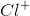Electron Configuration
AP Chemistry · Learn by Concept
Help Questions
AP Chemistry › Electron Configuration
1 - 1
1
Which of the following has the electron configuration of ![[Ne]3s^23p^6](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1074018/gif.latex)
CORRECT
Explanation
The task is to find the atom or ion with its first three energy levels completely filled. Argon is not an answer choice, which means that it must be one of the ions with an atomic number close to argon's atomic number. Recalling that a positive charge means electrons were lost from the outermost shell and that a negative charge means electrons were gained in the outermost shell, the only answer that works is 
AI Study CoachYour personal study buddy
Instant TutoringConnect with a tutor now
Request a TutorGet matched with an expert
Live ClassesJoin interactive classes
Question of the DayDaily practice to build your skills
FlashcardsStudy and memorize key concepts
WorksheetsBuild custom practice quizzes
AI SolverStep-by-step problem solutions
Learn by ConceptMaster concepts step by step
Diagnostic TestsAssess your knowledge level
Practice TestsTest your skills with exam questions
GamesLearn through free games