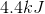Phase Changes
Physical Chemistry · Learn by Concept
Help Questions
Physical Chemistry › Phase Changes
1 - 1
1
What amount of heat must be added to a 20-gram sample of ice in order to raise the temperature from 

CORRECT
Explanation
This question requires three steps:
1. We need to raise the temperature of the ice from 

2. We need to melt the ice.
3. We need to raise the temperature of the water from 

Steps one and three can use the equation:
Step 2 requires the enthalpy of fusion to be multiplied by the mass of the water sample.
Finding these three added heats will give us the total amount of heat needed to raise the temperature from 

AI Study CoachYour personal study buddy
Instant TutoringConnect with a tutor now
Request a TutorGet matched with an expert
Live ClassesJoin interactive classes
Question of the DayDaily practice to build your skills
FlashcardsStudy and memorize key concepts
WorksheetsBuild custom practice quizzes
AI SolverStep-by-step problem solutions
Learn by ConceptMaster concepts step by step
Diagnostic TestsAssess your knowledge level
Practice TestsTest your skills with exam questions
GamesLearn through free games