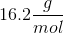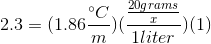Freezing Point
Physical Chemistry · Learn by Concept
Help Questions
Physical Chemistry › Freezing Point
1 - 1
1
When 20 grams of a solute is added to a liter of water, the freezing point becomes 
Assume that the solute does not make ions in solution.
CORRECT
Explanation
We can use the freezing point depression equation in order to determine the molar mass of the unknown solute.
AI Study CoachYour personal study buddy
Instant TutoringConnect with a tutor now
Request a TutorGet matched with an expert
Live ClassesJoin interactive classes
Question of the DayDaily practice to build your skills
FlashcardsStudy and memorize key concepts
WorksheetsBuild custom practice quizzes
AI SolverStep-by-step problem solutions
Learn by ConceptMaster concepts step by step
Diagnostic TestsAssess your knowledge level
Practice TestsTest your skills with exam questions
GamesLearn through free games