Organic Chemistry, Biochemistry, and Metabolism - MCAT Biological and Biochemical Foundations of Living Systems
Card 1 of 2928
What would charge would you expect on alanine when placed in a solution with a pH of 1.00?
What would charge would you expect on alanine when placed in a solution with a pH of 1.00?
Tap to reveal answer
Since alanine is nonpolar, we know that the only parts of the amino acid that can be charged are the N-terminus and the C-terminus.
In an acidic solution, there is an excessive amount of protons available to protonate the amino acid. As a result, the carboxylic acid end and the amine end will both be fully protonated. This will result in an overall charge of +1, due to the nitrogen having three hydrogens attached.
Since alanine is nonpolar, we know that the only parts of the amino acid that can be charged are the N-terminus and the C-terminus.
In an acidic solution, there is an excessive amount of protons available to protonate the amino acid. As a result, the carboxylic acid end and the amine end will both be fully protonated. This will result in an overall charge of +1, due to the nitrogen having three hydrogens attached.
← Didn't Know|Knew It →
Which of the following components is not found in a nucleotide?
Which of the following components is not found in a nucleotide?
Tap to reveal answer
Nucleotides are composed of a pentose sugar, phosphate group, and nitrogenous base. Nucleotides are the building blocks of nucleic acids, such as DNA and RNA. Phosphodiester bonds form between the phosphate group of one nucleotide and the 3' carbon of the pentose sugar on a second nucleotide to form a linkage. A nucleoside describes the molecule that is formed by a pentose sugar and nitrogenous base.
Sulfate groups are not found in nucleotides or nucleosides.
Nucleotides are composed of a pentose sugar, phosphate group, and nitrogenous base. Nucleotides are the building blocks of nucleic acids, such as DNA and RNA. Phosphodiester bonds form between the phosphate group of one nucleotide and the 3' carbon of the pentose sugar on a second nucleotide to form a linkage. A nucleoside describes the molecule that is formed by a pentose sugar and nitrogenous base.
Sulfate groups are not found in nucleotides or nucleosides.
← Didn't Know|Knew It →
Which of the following amino acids contain(s) a hydrophilic functional group in its side chain?
I. Serine
II. Valine
III. Phenylalanine
IV. Tyrosine
V. Threonine
Which of the following amino acids contain(s) a hydrophilic functional group in its side chain?
I. Serine
II. Valine
III. Phenylalanine
IV. Tyrosine
V. Threonine
Tap to reveal answer
Serine and threonine are classified as hydrophilic amino acids and contain hydroxyl (-OH) groups in their side chains. Tyrosine, although it is considered hydrophobic, does contain a hydrophilic hydroxyl group in its side chain. The answer is I, IV, and V, as all of these contain hydrophilic functional groups.
Serine and threonine are classified as hydrophilic amino acids and contain hydroxyl (-OH) groups in their side chains. Tyrosine, although it is considered hydrophobic, does contain a hydrophilic hydroxyl group in its side chain. The answer is I, IV, and V, as all of these contain hydrophilic functional groups.
← Didn't Know|Knew It →
Which of the following amino acid sequences would be found on the cytoplasm side of a transmembrane protein?
Which of the following amino acid sequences would be found on the cytoplasm side of a transmembrane protein?
Tap to reveal answer
Transmembrane proteins are embedded within the phospholipid bilayer of the cell membrane; therefore, the protein is exposed to the nonpolar fatty acid tails, the polar phospholipid heads, and the polar environments of the cytoplasm and extracellular space.
The questions asks which amino acids would be found facing the cytoplasm. Because the cytoplasm is polar, the amino acids interacting with the cytoplasm must also be polar. Glutamine, threonine, and tyrosine are all polar amino acids, making this the best answer. The other answers contain nonpolar amino acids (proline, leucine, cysteine, valine) causing these answers to be incorrect.
Transmembrane proteins are embedded within the phospholipid bilayer of the cell membrane; therefore, the protein is exposed to the nonpolar fatty acid tails, the polar phospholipid heads, and the polar environments of the cytoplasm and extracellular space.
The questions asks which amino acids would be found facing the cytoplasm. Because the cytoplasm is polar, the amino acids interacting with the cytoplasm must also be polar. Glutamine, threonine, and tyrosine are all polar amino acids, making this the best answer. The other answers contain nonpolar amino acids (proline, leucine, cysteine, valine) causing these answers to be incorrect.
← Didn't Know|Knew It →
All amino acids have at least two pKa values, one corresponding to the carboxylic acid, and one corresponding to the amine functionality. Some amino acids with polar side chains also have a pKa associated with the sidechain functionality.
Phenylalanine has pKa values of 2.58 (carboxylic acid) and 9.24 (NH2).
Arginine has pKa values of 2.01 (carboxylic acid), 9.04 (NH2), and 12.48 (side chain).
Valine has pKa values of 2.29 (carboxylic acid) and 9.72 (NH2).
For this problem, consider a molecule made of up of three amino acids, as described below.
HO-phenylalanine-arginine-valine-NH2
What would the overall charge of this molecule be at a pH of 7?
All amino acids have at least two pKa values, one corresponding to the carboxylic acid, and one corresponding to the amine functionality. Some amino acids with polar side chains also have a pKa associated with the sidechain functionality.
Phenylalanine has pKa values of 2.58 (carboxylic acid) and 9.24 (NH2).
Arginine has pKa values of 2.01 (carboxylic acid), 9.04 (NH2), and 12.48 (side chain).
Valine has pKa values of 2.29 (carboxylic acid) and 9.72 (NH2).
For this problem, consider a molecule made of up of three amino acids, as described below.
HO-phenylalanine-arginine-valine-NH2
What would the overall charge of this molecule be at a pH of 7?
Tap to reveal answer
Phenylalanine:
Since the phenylalanine residue is at the C-terminus end of the molecule, only its carboxylic acid pKa is relevant, as its amine is involved in a peptide bond with arginine. At a pH of 7 (well above the carboxylic acid pKa of 2.58), the C-terminus carboxylic acid would be deprotonated and have a charge of  .
.
Arginine:
For the middle amino acid, arginine, the only relevant pKa is that of its side chain since both its carboxylic acid and amino groups are involved in peptide bonds with neighboring amino acids. Since the side chain of arginine would be protonated at a pH of 7 (well below the sidechain pKa of 12.48), this amino acid would have a charge of  .
.
Valine:
Finally, for valine, the relevant pKa to consider is the NH2 pKa of 9.72. At pH 7, this would also be protonated, resulting in a charge of  .
.
The overall charge of the molecule at pH 7 would be  .
.
Phenylalanine:
Since the phenylalanine residue is at the C-terminus end of the molecule, only its carboxylic acid pKa is relevant, as its amine is involved in a peptide bond with arginine. At a pH of 7 (well above the carboxylic acid pKa of 2.58), the C-terminus carboxylic acid would be deprotonated and have a charge of 
Arginine:
For the middle amino acid, arginine, the only relevant pKa is that of its side chain since both its carboxylic acid and amino groups are involved in peptide bonds with neighboring amino acids. Since the side chain of arginine would be protonated at a pH of 7 (well below the sidechain pKa of 12.48), this amino acid would have a charge of 
Valine:
Finally, for valine, the relevant pKa to consider is the NH2 pKa of 9.72. At pH 7, this would also be protonated, resulting in a charge of 
The overall charge of the molecule at pH 7 would be 
← Didn't Know|Knew It →
Which of the following amino acids is considered basic?
Which of the following amino acids is considered basic?
Tap to reveal answer
Basic amino acids are those containing an amine group, while acidic contain a carboxylic acid group.
The basic amino acids are lysine (the correct answer), arginine, and histidine.
The acidic amino acids are glutamic acid (glutamate) and aspartic acid (aspartate).
Basic amino acids are those containing an amine group, while acidic contain a carboxylic acid group.
The basic amino acids are lysine (the correct answer), arginine, and histidine.
The acidic amino acids are glutamic acid (glutamate) and aspartic acid (aspartate).
← Didn't Know|Knew It →
Proteins can have a maximum of four levels of structure: primary, secondary, tertiary, and quaternary. Although the proteins can spontaneously fold to a functional conformation, there are a variety of denaturing agents that can be used to disrupt the folding strategies of proteins. Mercaptoethanol is an example of a protein denaturing agent; its mechanism for dismantling proteins is to disrupt the disulfide bonds found in the protein. When urea is introduced to a protein, the hydrogen bonds holding the protein together are disrupted. Heat can also be considered a denaturing agent, which has the potential to disrupt all intermolecular interactions in a protein.
Which of the following proteins would be least affected by the introduction of mercaptoethanol?
Proteins can have a maximum of four levels of structure: primary, secondary, tertiary, and quaternary. Although the proteins can spontaneously fold to a functional conformation, there are a variety of denaturing agents that can be used to disrupt the folding strategies of proteins. Mercaptoethanol is an example of a protein denaturing agent; its mechanism for dismantling proteins is to disrupt the disulfide bonds found in the protein. When urea is introduced to a protein, the hydrogen bonds holding the protein together are disrupted. Heat can also be considered a denaturing agent, which has the potential to disrupt all intermolecular interactions in a protein.
Which of the following proteins would be least affected by the introduction of mercaptoethanol?
Tap to reveal answer
Disulfide bonds are disrupted by the introduction of mercaptoethanol. Disulfide bonds are created by the interaction of two cysteine amino acids on different parts of the amino acid chain during the development of tertiary protein folding. As a result, a protein with few to no cysteine amino acids would be least affected by mercaptoethanol.
Alpha-helices are linked to secondary structure, and do not involve disulfide bonds. Similarly, quaternary structure is not determined by disulfide bonds; a protein without quaternary structure could still have disulfide bonds, which would be disrupted by mercaptoethanol. Proline is not involved in disulfide bonds, and its frequency would not affect the potency of mercaptoethanol to the protein.
Disulfide bonds are disrupted by the introduction of mercaptoethanol. Disulfide bonds are created by the interaction of two cysteine amino acids on different parts of the amino acid chain during the development of tertiary protein folding. As a result, a protein with few to no cysteine amino acids would be least affected by mercaptoethanol.
Alpha-helices are linked to secondary structure, and do not involve disulfide bonds. Similarly, quaternary structure is not determined by disulfide bonds; a protein without quaternary structure could still have disulfide bonds, which would be disrupted by mercaptoethanol. Proline is not involved in disulfide bonds, and its frequency would not affect the potency of mercaptoethanol to the protein.
← Didn't Know|Knew It →
Which of the following forms of valine would be expected to exist under extremely acidic conditions?
Which of the following forms of valine would be expected to exist under extremely acidic conditions?
Tap to reveal answer
At low pH levels, we expect amino acids to exist in their cationic form. At a pH level equal to the isoelectric point (pI) we expect amino acids to exist as zwitterions, and at high pH levels we expect them to exist in their anionic forms.
Low pH causes protonation of the amino groups; high pH causes deprotonation of the carboxyl groups.
At low pH levels, we expect amino acids to exist in their cationic form. At a pH level equal to the isoelectric point (pI) we expect amino acids to exist as zwitterions, and at high pH levels we expect them to exist in their anionic forms.
Low pH causes protonation of the amino groups; high pH causes deprotonation of the carboxyl groups.
← Didn't Know|Knew It →
How many moles of base are required to fully deprotonate glutamic acid in its most acidic form (shown below)?

How many moles of base are required to fully deprotonate glutamic acid in its most acidic form (shown below)?

Tap to reveal answer
One mole is base is required to deprotonate each carboxylic acid or amine, until the amino acid exists in its most deprotonated and basic form. First, two moles of base would be needed to deprotonate the two acid groups on glutamic acid (one depicted on each end). Next, a third mole would be needed to deprotonate the amine to its neutral state. Three total moles is the correct answer.
One mole is base is required to deprotonate each carboxylic acid or amine, until the amino acid exists in its most deprotonated and basic form. First, two moles of base would be needed to deprotonate the two acid groups on glutamic acid (one depicted on each end). Next, a third mole would be needed to deprotonate the amine to its neutral state. Three total moles is the correct answer.
← Didn't Know|Knew It →
Polypeptides are molecules that contain multiple .
Polypeptides are molecules that contain multiple .
Tap to reveal answer
Polypeptides are made from individual amino acids through formation of peptide bonds.
Monosaccharides are the fundamental units for carbohydrates, while fatty acids come together to form lipids. Nucleotides and phosphates are key components of the nucleic acids, RNA and DNA.
Polypeptides are made from individual amino acids through formation of peptide bonds.
Monosaccharides are the fundamental units for carbohydrates, while fatty acids come together to form lipids. Nucleotides and phosphates are key components of the nucleic acids, RNA and DNA.
← Didn't Know|Knew It →
What type of amino acid will have an isoelectric point above 7?
What type of amino acid will have an isoelectric point above 7?
Tap to reveal answer
The isoelectric point is the pH where the amino acid solution is electrically neutral. In acidic conditions, the carboxylic acid and amino terminus will both be protonated. This results in a positive charge (due to the amine being protonated). In basic conditions, both ends are deprotonated, resulting in a negative charge.
If an amino acid is basic, that means that the pH must be above 7 in order to deprotonate the amine in the side chain. Only then will the amino acid be electrically neutral. All basic amino acids (three of them) have an isoelectric point above a pH of 7. All other amino acids have an isoelectric point at a pH below 7.
The isoelectric point is the pH where the amino acid solution is electrically neutral. In acidic conditions, the carboxylic acid and amino terminus will both be protonated. This results in a positive charge (due to the amine being protonated). In basic conditions, both ends are deprotonated, resulting in a negative charge.
If an amino acid is basic, that means that the pH must be above 7 in order to deprotonate the amine in the side chain. Only then will the amino acid be electrically neutral. All basic amino acids (three of them) have an isoelectric point above a pH of 7. All other amino acids have an isoelectric point at a pH below 7.
← Didn't Know|Knew It →
A polar amino acid in a highly basic solution is titrated with a strong acid. When will exactly half of the amino acid molecules be negatively charged?
A polar amino acid in a highly basic solution is titrated with a strong acid. When will exactly half of the amino acid molecules be negatively charged?
Tap to reveal answer
The amino acid is polar, so we do not need to worry about charges in the side chain. Since the amino acid is starting in a highly basic solution, we know that the amino acid is deprotonated at both termini. That is, the amino terminus is neutral and the carboxyl terminus is negative. This results in a net charge of -1 (at the carboxyl end). The first half equivalence point upon titration will be seen when half of the amino acids are neutral and half of the amino acids are negatively charged. The full first equivalence point will show all molecules with a neutral charge, while the second full equivalence point will show all molecules with a positive charge due to protonation of the amine.
The amino acid is polar, so we do not need to worry about charges in the side chain. Since the amino acid is starting in a highly basic solution, we know that the amino acid is deprotonated at both termini. That is, the amino terminus is neutral and the carboxyl terminus is negative. This results in a net charge of -1 (at the carboxyl end). The first half equivalence point upon titration will be seen when half of the amino acids are neutral and half of the amino acids are negatively charged. The full first equivalence point will show all molecules with a neutral charge, while the second full equivalence point will show all molecules with a positive charge due to protonation of the amine.
← Didn't Know|Knew It →
A polar amino acid in a highly basic solution is titrated with a strong acid. Which pH is the best prediction for the isoelectric point of this amino acid?
A polar amino acid in a highly basic solution is titrated with a strong acid. Which pH is the best prediction for the isoelectric point of this amino acid?
Tap to reveal answer
Only basic amino acids have an isoelectric point above a pH of 7. All others will have an isoelectric point below a pH of 7.
Since the amino acid is polar, it will have an isoelectric point below a pH of 7. Since a pH of 1 is extremely acidic, we would expect both ends of the amino acid to be protonated at that pH. As a result, a pH of 5.4 is more appropriate when predicting the amino acid's isoelectric point.
Only basic amino acids have an isoelectric point above a pH of 7. All others will have an isoelectric point below a pH of 7.
Since the amino acid is polar, it will have an isoelectric point below a pH of 7. Since a pH of 1 is extremely acidic, we would expect both ends of the amino acid to be protonated at that pH. As a result, a pH of 5.4 is more appropriate when predicting the amino acid's isoelectric point.
← Didn't Know|Knew It →
A researcher stains a transmembrane protein. The membrane-spanning region of the protein is stained red, whereas the other regions are stained blue. Which of the following will you most likely find in the red region?
I. Glycine, which has a side chain of 
II. Cysteine, which has a side chain of 
III. Alanine, which has a side chain of 
A researcher stains a transmembrane protein. The membrane-spanning region of the protein is stained red, whereas the other regions are stained blue. Which of the following will you most likely find in the red region?
I. Glycine, which has a side chain of
II. Cysteine, which has a side chain of
III. Alanine, which has a side chain of
Tap to reveal answer
The question states that the membrane-spanning region of the transmembrane protein is stained red; therefore, you will find only hydrophobic amino acids in this region. Recall that an amino acid has a central carbon that has a hydrogen group, carboxylic acid group, amino group, and a side chain attached. The differences between amino acids arise from the different side chains.
A hydrophobic amino acid contains nonpolar side chains. Glycine and alanine contain  and
and  side chains, respectively. Both of these amino acids will have nonpolar properties and be found in the red region of the protein. Cysteine contains
side chains, respectively. Both of these amino acids will have nonpolar properties and be found in the red region of the protein. Cysteine contains  , which is a polar side chain, and will be found in the blue region.
, which is a polar side chain, and will be found in the blue region.
The question states that the membrane-spanning region of the transmembrane protein is stained red; therefore, you will find only hydrophobic amino acids in this region. Recall that an amino acid has a central carbon that has a hydrogen group, carboxylic acid group, amino group, and a side chain attached. The differences between amino acids arise from the different side chains.
A hydrophobic amino acid contains nonpolar side chains. Glycine and alanine contain 


← Didn't Know|Knew It →
Sildenafil (commonly called Viagra) is a common drug used to treat erectile dysfunction and pulmonary arterial hypertension. Sildenafil's effect comes from its ability to cause vasodilation in smooth muscle cells. For this problem, we're only going to consider its effects on erections in males.
Erectile dysfunction is a common medical problem in older men. Its most significant effect is the prevention of erections. Erections occur when there is an increase in blood flow via enlargement of an artery (vasodilation). Understanding the mechanism by which vasodilations occur is important in order to treat erectile dysfunction.
Erections occur when nitric oxide  is released from an area in the penis and binds to guanylate cyclase in other cells of the penis, which creates cyclic guanosine monophosphate (cGMP) from GTP. cGMP causes a relaxation of the arterial wall in order to increase blood flow to the region, thereby causing an erection. cGMP is broken down over time by cGMP-specific phosphodiesterase type 5 (PDE5) into GTP, which reverses the effect and causes vasoconstriction on the arterial wall. Combatting this effect is the major method by which Viagra functions.
is released from an area in the penis and binds to guanylate cyclase in other cells of the penis, which creates cyclic guanosine monophosphate (cGMP) from GTP. cGMP causes a relaxation of the arterial wall in order to increase blood flow to the region, thereby causing an erection. cGMP is broken down over time by cGMP-specific phosphodiesterase type 5 (PDE5) into GTP, which reverses the effect and causes vasoconstriction on the arterial wall. Combatting this effect is the major method by which Viagra functions.
Which of the following amino acids is most likely used to synthesize nitric oxide?
Sildenafil (commonly called Viagra) is a common drug used to treat erectile dysfunction and pulmonary arterial hypertension. Sildenafil's effect comes from its ability to cause vasodilation in smooth muscle cells. For this problem, we're only going to consider its effects on erections in males.
Erectile dysfunction is a common medical problem in older men. Its most significant effect is the prevention of erections. Erections occur when there is an increase in blood flow via enlargement of an artery (vasodilation). Understanding the mechanism by which vasodilations occur is important in order to treat erectile dysfunction.
Erections occur when nitric oxide 
Which of the following amino acids is most likely used to synthesize nitric oxide?
Tap to reveal answer
Let's consider nitric oxide, which has the chemical formula  .
.
In most biological contexts, oxygen is relatively easy to derive since it can be derived from lots of sources, such as water or air. However, the nitrogen in nitric oxide is the more difficult atom to obtain.
All of the listed amino acids have a backbone that has an amino group, but only one has nitrogen within its side chain, and that is arginine. Since arginine has 3 nitrogen atoms on its side chains, it is the most likely candidate to be stripped of its nitrogen to form nitric oxide.
Let's consider nitric oxide, which has the chemical formula 
In most biological contexts, oxygen is relatively easy to derive since it can be derived from lots of sources, such as water or air. However, the nitrogen in nitric oxide is the more difficult atom to obtain.
All of the listed amino acids have a backbone that has an amino group, but only one has nitrogen within its side chain, and that is arginine. Since arginine has 3 nitrogen atoms on its side chains, it is the most likely candidate to be stripped of its nitrogen to form nitric oxide.
← Didn't Know|Knew It →
The end product of an enzymatic reaction inhibits formation of product in an earlier step. This type of enzymatic regulation is known as .
The end product of an enzymatic reaction inhibits formation of product in an earlier step. This type of enzymatic regulation is known as .
Tap to reveal answer
Feedback inhibition is a type of regulation in which an enzyme product blocks an earlier part of a metabolic reaction. This allows cells to regulate resources by signaling when enough product is made.
Feedback inhibition is a type of regulation in which an enzyme product blocks an earlier part of a metabolic reaction. This allows cells to regulate resources by signaling when enough product is made.
← Didn't Know|Knew It →
An unknown molecule is added to an enzyme-catalyzed reaction, immediately decreasing its rate. If the addition of more substrate has no effect, but the addition of an antibody for the unknown molecule restores the initial reaction rate, what form of inhibition is most likely occurring?
An unknown molecule is added to an enzyme-catalyzed reaction, immediately decreasing its rate. If the addition of more substrate has no effect, but the addition of an antibody for the unknown molecule restores the initial reaction rate, what form of inhibition is most likely occurring?
Tap to reveal answer
This question is referring specifically to the different modes of enzyme inhibition. The given fact that increasing substrate concentration does not restore enzyme function indicates that the inhibitor is binding to the enzyme at an allosteric site (eliminating competitive inhibition). The given fact that inhibitor-specific antibodies restored enzyme function indicates that the inhibition is reversible.
Uncompetitive inhibition is a specific type of noncompetitive inhibition in which the inhibitior binds to the enzyme-substrate complex. We are unable to conclude that this is the case based on the given information alone.
This question is referring specifically to the different modes of enzyme inhibition. The given fact that increasing substrate concentration does not restore enzyme function indicates that the inhibitor is binding to the enzyme at an allosteric site (eliminating competitive inhibition). The given fact that inhibitor-specific antibodies restored enzyme function indicates that the inhibition is reversible.
Uncompetitive inhibition is a specific type of noncompetitive inhibition in which the inhibitior binds to the enzyme-substrate complex. We are unable to conclude that this is the case based on the given information alone.
← Didn't Know|Knew It →
Duchenne Muscular Dystrophy is an X-linked recessive genetic disorder, resulting in the loss of the dystrophin protein. In healthy muscle, dystrophin localizes to the sarcolemma and helps anchor the muscle fiber to the basal lamina. The loss of this protein results in progressive muscle weakness, and eventually death.
In the muscle fibers, the effects of the disease can be exacerbated by auto-immune interference. Weakness of the sarcolemma leads to damage and tears in the membrane. The body’s immune system recognizes the damage and attempts to repair it; however, since the damage exists as a chronic condition, leukocytes begin to present the damaged protein fragments as antigens, stimulating a targeted attack on the damaged parts of the muscle fiber. The attack causes inflammation, fibrosis, and necrosis, further weakening the muscle.
Studies have shown that despite the severe pathology of the muscle fibers, the innervation of the muscle is unaffected.
Which of the following would best describe the dystrophin protein?
Duchenne Muscular Dystrophy is an X-linked recessive genetic disorder, resulting in the loss of the dystrophin protein. In healthy muscle, dystrophin localizes to the sarcolemma and helps anchor the muscle fiber to the basal lamina. The loss of this protein results in progressive muscle weakness, and eventually death.
In the muscle fibers, the effects of the disease can be exacerbated by auto-immune interference. Weakness of the sarcolemma leads to damage and tears in the membrane. The body’s immune system recognizes the damage and attempts to repair it; however, since the damage exists as a chronic condition, leukocytes begin to present the damaged protein fragments as antigens, stimulating a targeted attack on the damaged parts of the muscle fiber. The attack causes inflammation, fibrosis, and necrosis, further weakening the muscle.
Studies have shown that despite the severe pathology of the muscle fibers, the innervation of the muscle is unaffected.
Which of the following would best describe the dystrophin protein?
Tap to reveal answer
The passage tells us that "dystrophin localizes to the sarcolemma," so we know it is located at the membrane of the muscle fiber. We also know that its role is to structurally link the muscle fiber and the basal lamina. We can eliminate the choices for ion channel, signaling protein, and chemical receptor based on what we know about dystrophin's function. We are left with either fibrous protein or transmembrane protein. Though fibrous proteins also have structural roles, transmembrane protein is the best choice because we know that dystrophin is linking the muscle fiber to another structure, meaning that it must span the membrane.
The passage tells us that "dystrophin localizes to the sarcolemma," so we know it is located at the membrane of the muscle fiber. We also know that its role is to structurally link the muscle fiber and the basal lamina. We can eliminate the choices for ion channel, signaling protein, and chemical receptor based on what we know about dystrophin's function. We are left with either fibrous protein or transmembrane protein. Though fibrous proteins also have structural roles, transmembrane protein is the best choice because we know that dystrophin is linking the muscle fiber to another structure, meaning that it must span the membrane.
← Didn't Know|Knew It →
Of the following statements, which is true regarding the change in free energy (ΔG) of a reaction?
Of the following statements, which is true regarding the change in free energy (ΔG) of a reaction?
Tap to reveal answer
Gibbs Free Energy (G) is a measure of the capacity of a system to do useful work as it proceeds to equilibrium. ΔG measures the spontaneity of a reaction; a negative value for ΔG indicates a spontaneous reaction, a positive value indicates a non-spontaneous reaction, and a value of zero indicates a reaction at equilibrium. ΔG does not predict enzyme kinetics; it only predicts thermodynamics, thus, two of the answers are correct.
Gibbs Free Energy (G) is a measure of the capacity of a system to do useful work as it proceeds to equilibrium. ΔG measures the spontaneity of a reaction; a negative value for ΔG indicates a spontaneous reaction, a positive value indicates a non-spontaneous reaction, and a value of zero indicates a reaction at equilibrium. ΔG does not predict enzyme kinetics; it only predicts thermodynamics, thus, two of the answers are correct.
← Didn't Know|Knew It →
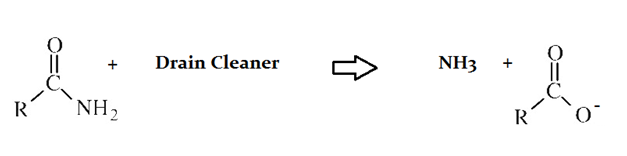
Drain cleaners a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
Protein that forms the hair discussed in the preceeding passage is considered strucutral protein. Functional proteins, such as enzymes, are the other major class. Which of the following is true of enzymes?
Drain cleaners a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
Protein that forms the hair discussed in the preceeding passage is considered strucutral protein. Functional proteins, such as enzymes, are the other major class. Which of the following is true of enzymes?
Tap to reveal answer
Enzymes are biological catalysts that function to lower activation energy via an alternative reaction pathway. They never alter the equilibrium of the reaction they impact.
Enzymes are biological catalysts that function to lower activation energy via an alternative reaction pathway. They never alter the equilibrium of the reaction they impact.
← Didn't Know|Knew It →