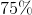Understanding Heat Engines
Physics · Learn by Concept
Help Questions
Physics › Understanding Heat Engines
1 - 1
1
A Carnot Cycle operates using a heat reservoir at a temperature of 


CORRECT
Explanation
The efficiency of a Carnot Cycle is defined by:
In this calculation, temperatures are given in Kelvin. Since Celsius and Kelvin exist in a one-to-one ratio, a conversion is unnecessary unless desired. We will perform the conversion, since most classes will advise it.
Using these values, we can calculate the efficiency:
Convert this value to a percentage:
AI Study CoachYour personal study buddy
Instant TutoringConnect with a tutor now
Request a TutorGet matched with an expert
Live ClassesJoin interactive classes
Question of the DayDaily practice to build your skills
FlashcardsStudy and memorize key concepts
WorksheetsBuild custom practice quizzes
AI SolverStep-by-step problem solutions
Learn by ConceptMaster concepts step by step
Diagnostic TestsAssess your knowledge level
Practice TestsTest your skills with exam questions
GamesLearn through free games