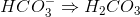Identifying and Defining Acids and Bases
AP Chemistry · Learn by Concept
Help Questions
AP Chemistry › Identifying and Defining Acids and Bases
1 - 1
1
Which of the following cannot act as a Bronsted-Lowry base (proton recipient) in aqueous solution?
CORRECT
Explanation



AI Study CoachYour personal study buddy
Instant TutoringConnect with a tutor now
Request a TutorGet matched with an expert
Live ClassesJoin interactive classes
Question of the DayDaily practice to build your skills
FlashcardsStudy and memorize key concepts
WorksheetsBuild custom practice quizzes
AI SolverStep-by-step problem solutions
Learn by ConceptMaster concepts step by step
Diagnostic TestsAssess your knowledge level
Practice TestsTest your skills with exam questions
GamesLearn through free games