Solutions and States of Matter - AP Chemistry
Card 1 of 2320
How much faster is the rate of effusion of  than the rate of effusion of
than the rate of effusion of  ?
?
How much faster is the rate of effusion of 

Tap to reveal answer
By Graham's Law,  . The molar mass of
. The molar mass of  is 2 g/mol and the molar mass of
is 2 g/mol and the molar mass of  is 18 g/mol. Thus,
is 18 g/mol. Thus, 
By Graham's Law, 


← Didn't Know|Knew It →
30 mL of 1.0 M  solution is diluted with water to a volume of 3 L. What is the new concentration of the solution?
solution is diluted with water to a volume of 3 L. What is the new concentration of the solution?
30 mL of 1.0 M 
Tap to reveal answer
Recall that  Thus, the volume of the solution increased from 30 mL to 3000 mL, which is a factor of 100. Thus, the new concentration must be 100 times less than the original concentration, and
Thus, the volume of the solution increased from 30 mL to 3000 mL, which is a factor of 100. Thus, the new concentration must be 100 times less than the original concentration, and  .
.
Recall that 

← Didn't Know|Knew It →
Consider the typical phase diagram of a compound given below.
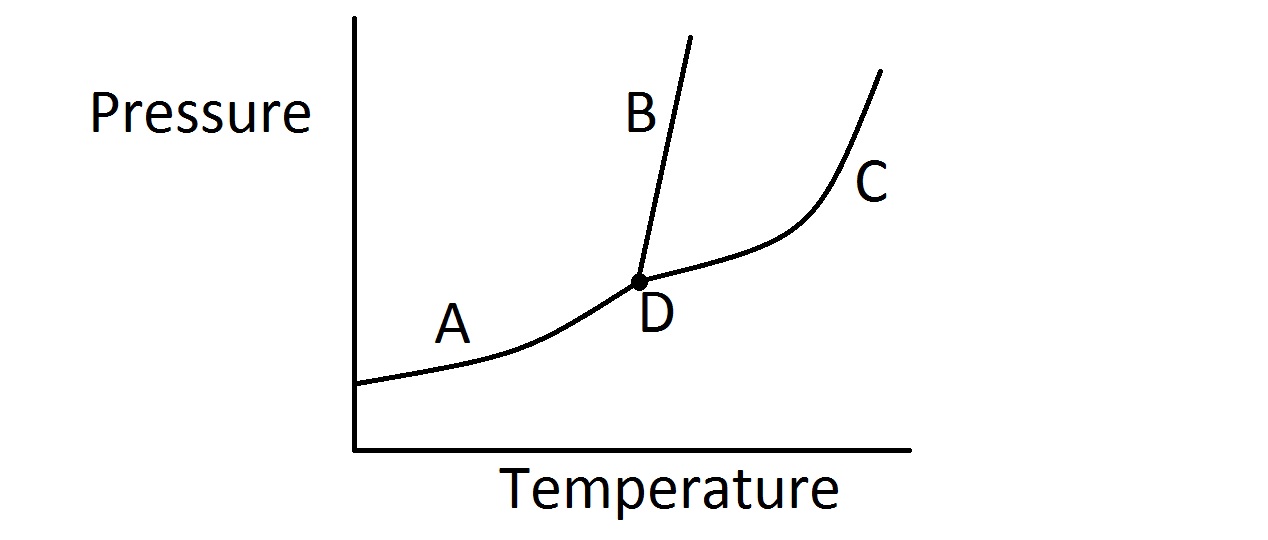
Which of the following lines or points on the diagram represents a situation in which the rate of vaporization of the compound is equal to its rate of condensation?
Consider the typical phase diagram of a compound given below.

Which of the following lines or points on the diagram represents a situation in which the rate of vaporization of the compound is equal to its rate of condensation?
Tap to reveal answer
In this question, we're presented with a phase diagram and are asked to determine where on the graph the rate of vaporization equals the rate of condensation.
First, it's important to realize that when the rate of vaporization and condensation are equal, we have an equilibrium of liquid and gas phases. In other words, for a given temperature and pressure, the rate at which the liquid evaporates into a gas is exactly equal to the rate at which the gas condenses into a liquid.
On a phase diagram, the area of the upper left portion of the diagram represents the solid state. The middle portion of the diagram represents the liquid state. The bottom and right most part of the diagram represents the gas phase.
Furthermore, each line on the diagram represents the specific combination of temperature and pressure in which a given compound will exist in equilibrium between two phases. The point where all three lines intersect, however, represents the triple point. This tells us the temperature and pressure in which the compound will exist in an equilibrium between all three states.
Because we are looking for the equilibrium line that represents equilibrium of vaporization and condensation, we want the line that separates the liquid portion of the diagram from the gas portion. Based on the identification of regions on the diagram discussed above, that would be line C as shown in the diagram. Line A represents equilibrium between solid and gas (sublimation rate = deposition rate). Line B represents equilibrium between solid and liquid (melting rate = freezing rate).
In this question, we're presented with a phase diagram and are asked to determine where on the graph the rate of vaporization equals the rate of condensation.
First, it's important to realize that when the rate of vaporization and condensation are equal, we have an equilibrium of liquid and gas phases. In other words, for a given temperature and pressure, the rate at which the liquid evaporates into a gas is exactly equal to the rate at which the gas condenses into a liquid.
On a phase diagram, the area of the upper left portion of the diagram represents the solid state. The middle portion of the diagram represents the liquid state. The bottom and right most part of the diagram represents the gas phase.
Furthermore, each line on the diagram represents the specific combination of temperature and pressure in which a given compound will exist in equilibrium between two phases. The point where all three lines intersect, however, represents the triple point. This tells us the temperature and pressure in which the compound will exist in an equilibrium between all three states.
Because we are looking for the equilibrium line that represents equilibrium of vaporization and condensation, we want the line that separates the liquid portion of the diagram from the gas portion. Based on the identification of regions on the diagram discussed above, that would be line C as shown in the diagram. Line A represents equilibrium between solid and gas (sublimation rate = deposition rate). Line B represents equilibrium between solid and liquid (melting rate = freezing rate).
← Didn't Know|Knew It →
Which of the following solutions would be expected to have the highest osmotic pressure?
Which of the following solutions would be expected to have the highest osmotic pressure?
Tap to reveal answer
In this question, we're asked to identify an answer choice that would be expected to give us a solution with the greatest osmotic pressure. Remember that osmotic pressure is proportional to the total number of dissolved solute particles in solution, regardless of the identity of those solute particles.
When looking at the answer choices, we need to keep in mind two things. First, we need to recognize the numerical value given for the concentration of the compound given. Secondly, we need to identify if the compound shown is capable of dissociating in solution to give rise to even more solute particles. This is important, as it would affect the osmotic pressure.
 would be expected to have the largest osmotic pressure because, in total, this would be a
would be expected to have the largest osmotic pressure because, in total, this would be a  solution after dissociation occurs.
solution after dissociation occurs.
In this question, we're asked to identify an answer choice that would be expected to give us a solution with the greatest osmotic pressure. Remember that osmotic pressure is proportional to the total number of dissolved solute particles in solution, regardless of the identity of those solute particles.
When looking at the answer choices, we need to keep in mind two things. First, we need to recognize the numerical value given for the concentration of the compound given. Secondly, we need to identify if the compound shown is capable of dissociating in solution to give rise to even more solute particles. This is important, as it would affect the osmotic pressure.


← Didn't Know|Knew It →
Which of the following is not a colligative property?
Which of the following is not a colligative property?
Tap to reveal answer
Colligative properties are properties of solutions which depend on the number of dissolved particles in solution. The four main colligative properties are:
-
Freezing point depression: The presence of a solute lowers the freezing point of a solution as compared to that of the pure solvent.
-
Boiling point elevation: The presence of a solute increases the boiling point of a solution as compared to that of the pure solvent.
-
Vapor pressure depression: The vapor pressure of a pure solvent is greater than that of a solution containing a non-volatile liquid. The lowering of vapor pressure leads to boiling point elevation.
-
Osmotic pressure: The osmotic pressure of a solution is the pressure difference between the solution and pure solvent when the two are in equilibrium across a semipermeable membrane. Because it depends on the concentration of solute particles in solution, it is a colligative property.
Electronegativity is not a property of solutions reliant on the number of dissolved particles, but a property of atoms themselves.
Colligative properties are properties of solutions which depend on the number of dissolved particles in solution. The four main colligative properties are:
-
Freezing point depression: The presence of a solute lowers the freezing point of a solution as compared to that of the pure solvent.
-
Boiling point elevation: The presence of a solute increases the boiling point of a solution as compared to that of the pure solvent.
-
Vapor pressure depression: The vapor pressure of a pure solvent is greater than that of a solution containing a non-volatile liquid. The lowering of vapor pressure leads to boiling point elevation.
-
Osmotic pressure: The osmotic pressure of a solution is the pressure difference between the solution and pure solvent when the two are in equilibrium across a semipermeable membrane. Because it depends on the concentration of solute particles in solution, it is a colligative property.
Electronegativity is not a property of solutions reliant on the number of dissolved particles, but a property of atoms themselves.
← Didn't Know|Knew It →
Which of the following aqueous solutions would be expected to have the greatest increase in boiling point?
Which of the following aqueous solutions would be expected to have the greatest increase in boiling point?
Tap to reveal answer
This question is asking us to identify a solution that increases the boiling point of water by the greatest amount.
To answer this, we need to understand the concept of colligative properties. When a solute dissolves in a solvent such as water, various physical properties are affected. The four colligative properties that change as a result of the addition of solute are freezing point, boiling point, vapor pressure, and osmotic pressure.
With regards to boiling point, as more solute is added to a solution, the boiling point increases. This is due to the fact that addition of solute makes it more difficult for the solute molecules to gain enough kinetic energy at the solution's surface to escape as a gas.
Furthermore, the identity of the solute does not matter. Thus, we need to look only at the number of dissolved solute particles rather than their identity. A compound such as sucrose will not dissociate in solution, which means that the osmotic pressure of the solution is the same as the concentration of sucrose.
Compounds that can dissociate into two or more particles will increase the osmolarity of the solution further. In this case,  will double the stated osmolarity.
will double the stated osmolarity.  , on the other hand, will dissociate completely because it is a strong acid, however the protons will not contribute to the osmolarity.
, on the other hand, will dissociate completely because it is a strong acid, however the protons will not contribute to the osmolarity.
 is able to dissociate into three equivalents of particles in solution. Thus, its initial concentration will be tripled, which gives it the highest osmolarity of any of the choices shown and will thus increase the boiling point by the greatest amount.
is able to dissociate into three equivalents of particles in solution. Thus, its initial concentration will be tripled, which gives it the highest osmolarity of any of the choices shown and will thus increase the boiling point by the greatest amount.
This question is asking us to identify a solution that increases the boiling point of water by the greatest amount.
To answer this, we need to understand the concept of colligative properties. When a solute dissolves in a solvent such as water, various physical properties are affected. The four colligative properties that change as a result of the addition of solute are freezing point, boiling point, vapor pressure, and osmotic pressure.
With regards to boiling point, as more solute is added to a solution, the boiling point increases. This is due to the fact that addition of solute makes it more difficult for the solute molecules to gain enough kinetic energy at the solution's surface to escape as a gas.
Furthermore, the identity of the solute does not matter. Thus, we need to look only at the number of dissolved solute particles rather than their identity. A compound such as sucrose will not dissociate in solution, which means that the osmotic pressure of the solution is the same as the concentration of sucrose.
Compounds that can dissociate into two or more particles will increase the osmolarity of the solution further. In this case, 


← Didn't Know|Knew It →
Per Graham's law of effusion, how does the molar mass relate to both the rate and time of effusion?
Per Graham's law of effusion, how does the molar mass relate to both the rate and time of effusion?
Tap to reveal answer

This equation explicitly shows how the rate of effusion is inversely proportional to the molar mass of a gas in a gaseous solution.
Because  , time relates to molar mass by:
, time relates to molar mass by:

Simplifying this equation, we see that:

As a result, time relates directly to molar mass
This equation explicitly shows how the rate of effusion is inversely proportional to the molar mass of a gas in a gaseous solution.
Because 
Simplifying this equation, we see that:
As a result, time relates directly to molar mass
← Didn't Know|Knew It →
At the same temperature, an unknown gas effuses at a rate that is  times that of oxygen. Find the molar mass, in grams per mole, of the unknown gas.
times that of oxygen. Find the molar mass, in grams per mole, of the unknown gas.
At the same temperature, an unknown gas effuses at a rate that is 
Tap to reveal answer
Recall Graham's Law of Effusion for two gases, A and B:

From the equation, we know the following:

Thus, we can solve for the molar mass of the unknown gas. Let  be the molar mass of the unknown gas.
be the molar mass of the unknown gas.



Make sure that your answer has  significant figures.
significant figures.
Recall Graham's Law of Effusion for two gases, A and B:
From the equation, we know the following:
Thus, we can solve for the molar mass of the unknown gas. Let 
Make sure that your answer has 
← Didn't Know|Knew It →
Which of the following is a true statement with regards to the relative effusion rates of oxygen and carbon dioxide?
Which of the following is a true statement with regards to the relative effusion rates of oxygen and carbon dioxide?
Tap to reveal answer
We're being asked to compare the effusion rates of oxygen and carbon dioxide.
Remember that effusion is the spontaneous movement of a gas through a small hole from one area to another. It's worth noting that at a given temperature, the average speed of all gas molecules in a system is used to calculate the average kinetic energy of the gas particles. This dependence of kinetic energy on temperature means that at a given temperature, any gas particle will have the same kinetic energy.
In this case, we can say that the kinetic energy of oxygen molecules in one system is equal to the kinetic energy of carbon dioxide molecules in another system. Furthermore, since mass is inversely proportional to velocity, identical kinetic energies would mean that as the mass of the gas particles in a system decreases, their velocity (and thus, effusion rates) would increase.
We can use this information to solve for the relative effusion rates between oxygen and carbon dioxide. By setting their kinetic energies equal to each other, we can derive an expression that relates their relative speeds to their relative masses.





Generally speaking, this expression shows how the velocity of any two gasses depends on their mass. In this case, the gasses are oxygen and carbon dioxide.
We can use the periodic table of the elements to find out the mass of each gas, and use that information to calculate the relative effusion rates.


This shows that oxygen will effuse at a rate that is about  faster than carbon dioxide.
faster than carbon dioxide.
We're being asked to compare the effusion rates of oxygen and carbon dioxide.
Remember that effusion is the spontaneous movement of a gas through a small hole from one area to another. It's worth noting that at a given temperature, the average speed of all gas molecules in a system is used to calculate the average kinetic energy of the gas particles. This dependence of kinetic energy on temperature means that at a given temperature, any gas particle will have the same kinetic energy.
In this case, we can say that the kinetic energy of oxygen molecules in one system is equal to the kinetic energy of carbon dioxide molecules in another system. Furthermore, since mass is inversely proportional to velocity, identical kinetic energies would mean that as the mass of the gas particles in a system decreases, their velocity (and thus, effusion rates) would increase.
We can use this information to solve for the relative effusion rates between oxygen and carbon dioxide. By setting their kinetic energies equal to each other, we can derive an expression that relates their relative speeds to their relative masses.
Generally speaking, this expression shows how the velocity of any two gasses depends on their mass. In this case, the gasses are oxygen and carbon dioxide.
We can use the periodic table of the elements to find out the mass of each gas, and use that information to calculate the relative effusion rates.
This shows that oxygen will effuse at a rate that is about 
← Didn't Know|Knew It →
A sample of Ne(g) effusses through a tiny hole in 60.7 s. An unknown gas, under identical conditions, effusses in 45.6 s.
What is the molar mass of the unknown gas?
A sample of Ne(g) effusses through a tiny hole in 60.7 s. An unknown gas, under identical conditions, effusses in 45.6 s.
What is the molar mass of the unknown gas?
Tap to reveal answer
To solve this problem use Graham's Law of Effusion



By plugging in the values we can rewrite the equation as



To solve this problem use Graham's Law of Effusion
By plugging in the values we can rewrite the equation as
← Didn't Know|Knew It →
Suppose that gas A effuses at a rate that is twice that of gas B. If the mass of gas A is halved and the mass of gas B is doubled, which of the following correctly describes the new relative effusion rates of these two gasses?
Suppose that gas A effuses at a rate that is twice that of gas B. If the mass of gas A is halved and the mass of gas B is doubled, which of the following correctly describes the new relative effusion rates of these two gasses?
Tap to reveal answer
For this question, we're given the relative effusion rates for two gasses. We're then told how the mass of each of these gasses is changed, and then we're asked to determine the new relative effusion rates of the two gasses.
First, we can recall the expression that describes the dependence of the effusion rates of two gasses on their mass. Since we're told that the rate of gas A is twice that of gas B, we can write the following expression.

Furthermore, since we're told that the mass of gas B is doubled and the mass of gas A is halved, we can determine how the rate will change.

Thus, we can see that the rate will change by a factor of two. Hence, the new rate will be  . Thus, gas A will now effuse at a rate
. Thus, gas A will now effuse at a rate  times that of gas B.
times that of gas B.
For this question, we're given the relative effusion rates for two gasses. We're then told how the mass of each of these gasses is changed, and then we're asked to determine the new relative effusion rates of the two gasses.
First, we can recall the expression that describes the dependence of the effusion rates of two gasses on their mass. Since we're told that the rate of gas A is twice that of gas B, we can write the following expression.
Furthermore, since we're told that the mass of gas B is doubled and the mass of gas A is halved, we can determine how the rate will change.
Thus, we can see that the rate will change by a factor of two. Hence, the new rate will be 

← Didn't Know|Knew It →
What is the energy of a photon that has a wavelength of 540 nm?
What is the energy of a photon that has a wavelength of 540 nm?
Tap to reveal answer
 ;
;  ;
; 


← Didn't Know|Knew It →
What transition would emit the longest wavelength of light?
What transition would emit the longest wavelength of light?
Tap to reveal answer
Wavelength is inversely proportional to energy. Since the transition from n=4 to n=3 is the lowest energy transition, it would have the longest wavelength.
Wavelength is inversely proportional to energy. Since the transition from n=4 to n=3 is the lowest energy transition, it would have the longest wavelength.
← Didn't Know|Knew It →
What photon wavelength can promote a transition from the n = 1 (ground state) to the n = 3 (excited state)?
What photon wavelength can promote a transition from the n = 1 (ground state) to the n = 3 (excited state)?
Tap to reveal answer

 ;
; 

← Didn't Know|Knew It →
A 3.0 eV light is shined on Gold ( Փ = 5.1 eV), silver (Փ =4.26 eV), Cesium (Փ = 2.1 eV), and Platinum (Փ = 6.35). If Փ is the work function of the metal, what metal would have electrons ejected?
A 3.0 eV light is shined on Gold ( Փ = 5.1 eV), silver (Փ =4.26 eV), Cesium (Փ = 2.1 eV), and Platinum (Փ = 6.35). If Փ is the work function of the metal, what metal would have electrons ejected?
Tap to reveal answer
The work function is the energy required to remove an electron from a metal. Only Cs metal has a work function less than the energy of the light.
The work function is the energy required to remove an electron from a metal. Only Cs metal has a work function less than the energy of the light.
← Didn't Know|Knew It →
What is the final pressure of a gas initially has a pressure of 10 atm at 50 L if the volume s now 25 L?
What is the final pressure of a gas initially has a pressure of 10 atm at 50 L if the volume s now 25 L?
Tap to reveal answer
Use P1V1 = P2V2
P1 = 10atm; V1 = 50L
P2 = X; V2 = 25L
(10atm)(50L) = (x)(25L)
500 = 25x
x = 20
Use P1V1 = P2V2
P1 = 10atm; V1 = 50L
P2 = X; V2 = 25L
(10atm)(50L) = (x)(25L)
500 = 25x
x = 20
← Didn't Know|Knew It →
Oil and vinegar is a very popular salad dressing. It also is commonly used for dipping bread. The main component of the oil phase is olive oil, while the main part of the vinegar portion is aqueous acetic acid.
When oil and vinegar salad dressing is allowed to stand at room temperature, two distinct phases are observed. The main explanation for this phenomenon is the difference in of the two phases.
Oil and vinegar is a very popular salad dressing. It also is commonly used for dipping bread. The main component of the oil phase is olive oil, while the main part of the vinegar portion is aqueous acetic acid.
When oil and vinegar salad dressing is allowed to stand at room temperature, two distinct phases are observed. The main explanation for this phenomenon is the difference in of the two phases.
Tap to reveal answer
Olive oil, like most oils, is non-polar, while aqueous acetic acid is very polar. These two phases do not mix because of their different solvent polarities.
Olive oil, like most oils, is non-polar, while aqueous acetic acid is very polar. These two phases do not mix because of their different solvent polarities.
← Didn't Know|Knew It →
A gas is behaving ideally
2 mols of the gas would have what volume at STP?
A gas is behaving ideally
2 mols of the gas would have what volume at STP?
Tap to reveal answer
use PV = nRT
V = nRT / P ; must covert T into K
V = (2)(0.0821)(273)/ 1
= 44.8 L
use PV = nRT
V = nRT / P ; must covert T into K
V = (2)(0.0821)(273)/ 1
= 44.8 L
← Didn't Know|Knew It →
Which of the following situations would most likely cause gases to deviate from ideal behavior?
Which of the following situations would most likely cause gases to deviate from ideal behavior?
Tap to reveal answer
At high pressure and low temperature two things are happening that will cause gases to deviate from ideal behavior. At low temperature the individual gas molecules are moving slower. As the pressure is increased the individual molecules are being pushed closer to one another. Thus, when the gas molecules are closer together and moving at reduced speeds they are more likely to interact with one another. Ideal gas behavior is dependent upon an absence of intermolecular interaction between the gas molecules. Therefore, the increased likelihood of intermolecular interactions between the gas molecules at increased pressure and decreased temperature is likely to cause gases to deviate from ideal behavior.
At high pressure and low temperature two things are happening that will cause gases to deviate from ideal behavior. At low temperature the individual gas molecules are moving slower. As the pressure is increased the individual molecules are being pushed closer to one another. Thus, when the gas molecules are closer together and moving at reduced speeds they are more likely to interact with one another. Ideal gas behavior is dependent upon an absence of intermolecular interaction between the gas molecules. Therefore, the increased likelihood of intermolecular interactions between the gas molecules at increased pressure and decreased temperature is likely to cause gases to deviate from ideal behavior.
← Didn't Know|Knew It →
Two balloons are filled with gas at STP. One is filled with hydrogen gas, the other with neon gas. The Volume of the balloon filled with hydrogen gas is 22.4 L, the balloon filled with neon is 44.8 L.
There are more atoms in which balloon?
Two balloons are filled with gas at STP. One is filled with hydrogen gas, the other with neon gas. The Volume of the balloon filled with hydrogen gas is 22.4 L, the balloon filled with neon is 44.8 L.
There are more atoms in which balloon?
Tap to reveal answer
Hydrogen gas is a diatomic gas, so the molecules that are filling the balloon exist as H2. Neon since it is a noble gas exists as a monoatomic gas, so the molecules that are filling the neon balloon exist as Ne. The volumes given allow us to calculate the amount of each gas in moles. At STP one mole of gas occupies 22.4 L, so there is one mole of hydrogen gas and there are two moles of neon gas. One mole of hydrogen gas indicates that there are actually two moles of hydrogen atoms in the balloon. Two moles of neon gas indicates that there are two moles of neon gas present in the balloon because neon exists as a monoatomic gas. Thus there are two moles of atoms in each balloon.
Hydrogen gas is a diatomic gas, so the molecules that are filling the balloon exist as H2. Neon since it is a noble gas exists as a monoatomic gas, so the molecules that are filling the neon balloon exist as Ne. The volumes given allow us to calculate the amount of each gas in moles. At STP one mole of gas occupies 22.4 L, so there is one mole of hydrogen gas and there are two moles of neon gas. One mole of hydrogen gas indicates that there are actually two moles of hydrogen atoms in the balloon. Two moles of neon gas indicates that there are two moles of neon gas present in the balloon because neon exists as a monoatomic gas. Thus there are two moles of atoms in each balloon.
← Didn't Know|Knew It →


