Gibbs Free Energy and Spontaneity - AP Chemistry
Card 1 of 208
What must be true of a spontaneous process?
What must be true of a spontaneous process?
Tap to reveal answer
Change in free energy must always be negative for a spontaneous process. Additionally, Q must be less than K so that the reaction will proceed in the forward reaction, toward equilibrium.
Change in free energy must always be negative for a spontaneous process. Additionally, Q must be less than K so that the reaction will proceed in the forward reaction, toward equilibrium.
← Didn't Know|Knew It →
If a reaction has a positive value for its enthalpy and a negative value for its entropy, which of the following is true?
If a reaction has a positive value for its enthalpy and a negative value for its entropy, which of the following is true?
Tap to reveal answer
Given the equation for free energy, (delta)G = (delta)H-T(delta)S, we can determine that the reaction is nonspontaneous at all temperatures if H is positive and S is negative. This combination would always lead to a positive G value, meaning that free energy is required for the reaction to take place and it is therefore nonspontaneous.
Given the equation for free energy, (delta)G = (delta)H-T(delta)S, we can determine that the reaction is nonspontaneous at all temperatures if H is positive and S is negative. This combination would always lead to a positive G value, meaning that free energy is required for the reaction to take place and it is therefore nonspontaneous.
← Didn't Know|Knew It →
Of the following reactions, which of the following is only spontaneous at high enough temperatures?
Of the following reactions, which of the following is only spontaneous at high enough temperatures?
Tap to reveal answer
Based on Gibbs Free Energy, ∆G = ∆H – T∆S, we find that ∆H– will contribute to spontaneity (∆G<0). Since we are looking at temperatures, in order for ∆S to make an nonspontaneous reaction spontaneous at only high temperatures, ∆H will be positive, and ∆S will be positive.
Based on Gibbs Free Energy, ∆G = ∆H – T∆S, we find that ∆H– will contribute to spontaneity (∆G<0). Since we are looking at temperatures, in order for ∆S to make an nonspontaneous reaction spontaneous at only high temperatures, ∆H will be positive, and ∆S will be positive.
← Didn't Know|Knew It →
Suppose that a rxn has ∆H = -28 kJ and ∆S= -60 J/K. At what temperature will it
change from spontaneous to non-spontaneous?
Suppose that a rxn has ∆H = -28 kJ and ∆S= -60 J/K. At what temperature will it
change from spontaneous to non-spontaneous?
Tap to reveal answer
Approximately 467 K. ∆G=∆H-T∆S and a rxn proceeds spontaneously when ∆G < 0
and is non-spontaneous when ∆G > 0. So if we set ∆G=0 and solve the equation for T, we will see that the crossover from spontaneous to non-spontaneous occurs when T=467K.
Approximately 467 K. ∆G=∆H-T∆S and a rxn proceeds spontaneously when ∆G < 0
and is non-spontaneous when ∆G > 0. So if we set ∆G=0 and solve the equation for T, we will see that the crossover from spontaneous to non-spontaneous occurs when T=467K.
← Didn't Know|Knew It →
Na(s) + 1/2 Cl2(g) → NaCl (s)
The Standard Heat of formation for NaCl is -411.1 kJ
Which of the following descriptions accurately describes the reaction above?
Na(s) + 1/2 Cl2(g) → NaCl (s)
The Standard Heat of formation for NaCl is -411.1 kJ
Which of the following descriptions accurately describes the reaction above?
Tap to reveal answer
A reaction being favorable or unfavorable is largely determined by the thermochemistry of the reaction. More specifically it is determined by the change in gibbs free energy which is determined by the change in enthalpy, the change in entropy, and the temperature of the reaction. Here we are told that the reaction has a favorable enthalpy change (- means energy is released). Qualitatively we can see that the reaction will have an unfavorable change in entropy because the product, being a solid, is more ordered than the reactants which are both solid and gaseous. Thus we can conclude that the reaction is favorable because of the favorable change in enthalpy, which helps to overcome the unfavorable change in entropy.
A reaction being favorable or unfavorable is largely determined by the thermochemistry of the reaction. More specifically it is determined by the change in gibbs free energy which is determined by the change in enthalpy, the change in entropy, and the temperature of the reaction. Here we are told that the reaction has a favorable enthalpy change (- means energy is released). Qualitatively we can see that the reaction will have an unfavorable change in entropy because the product, being a solid, is more ordered than the reactants which are both solid and gaseous. Thus we can conclude that the reaction is favorable because of the favorable change in enthalpy, which helps to overcome the unfavorable change in entropy.
← Didn't Know|Knew It →
If the reaction quotient (Q) is less than the equilibrium constant (K), what is true about the Gibbs free energy?
If the reaction quotient (Q) is less than the equilibrium constant (K), what is true about the Gibbs free energy?
Tap to reveal answer
If Q is less than K, then the reaction has not yet reached the equilibrium state. It will proceed spontaneously in the forward direction. Since it is proceeding spontaneously in the forward direction, this must mean that the ΔG (Gibbs free energy) must be negative, or less than zero.
Enthalpy and temperature are necessary when mathematically evaluating Gibbs free energy, but the reaction quotient and equilibrium constant provide enough information to qualitatively determine that the reaction is proceeding spontaneously.
If Q is less than K, then the reaction has not yet reached the equilibrium state. It will proceed spontaneously in the forward direction. Since it is proceeding spontaneously in the forward direction, this must mean that the ΔG (Gibbs free energy) must be negative, or less than zero.
Enthalpy and temperature are necessary when mathematically evaluating Gibbs free energy, but the reaction quotient and equilibrium constant provide enough information to qualitatively determine that the reaction is proceeding spontaneously.
← Didn't Know|Knew It →
If the reaction quotient (Q) is greater than the equilibrium constant (K), what is true about the Gibbs free energy?
If the reaction quotient (Q) is greater than the equilibrium constant (K), what is true about the Gibbs free energy?
Tap to reveal answer
If Q is greater than K, the reaction has exceeded the equilibrium state. It will proceed nonspontaneously (since equilibrium has already been reached), and this must mean that the ΔG (gibbs free energy) must be positive, or greater than zero.
If Q is greater than K, the reaction has exceeded the equilibrium state. It will proceed nonspontaneously (since equilibrium has already been reached), and this must mean that the ΔG (gibbs free energy) must be positive, or greater than zero.
← Didn't Know|Knew It →
Consider the following reaction:

At 298K,  ,
,  , and
, and  . Assuming that
. Assuming that  and
and  do not change with temperature, what is the value of
do not change with temperature, what is the value of  at 500K? Does the reaction become more or less spontaneous?
at 500K? Does the reaction become more or less spontaneous?
Consider the following reaction:
At 298K, 





Tap to reveal answer
We know that  , so
, so

There is a net decrease in free energy, so the reaction is more spontaneous at 500 K.
We know that 
There is a net decrease in free energy, so the reaction is more spontaneous at 500 K.
← Didn't Know|Knew It →
The entropy and enthalpy of a reaction are both negative. Is the reaction spontaneous?
The entropy and enthalpy of a reaction are both negative. Is the reaction spontaneous?
Tap to reveal answer
A reaction is spontaneous if the Gibb's Free Energy of the reaction is negative.

If  , the enthalpy, and
, the enthalpy, and  , the entropy, are both negative, then the reaction will be spontaneous if and only if the magnitude of the enthalpy is greater than the magnitude of the entropy times the temperature.
, the entropy, are both negative, then the reaction will be spontaneous if and only if the magnitude of the enthalpy is greater than the magnitude of the entropy times the temperature.
A reaction is spontaneous if the Gibb's Free Energy of the reaction is negative.
If 

← Didn't Know|Knew It →
Calcium carbonate is formed from calcium oxide and carbon dioxide.

Given that this reaction is spontaneous at low temperatures and non-spontaneous at high temperatures, what must be true about the change of enthalphy ( ) and the change of entropy (
) and the change of entropy (

 )?
)?
Calcium carbonate is formed from calcium oxide and carbon dioxide.
Given that this reaction is spontaneous at low temperatures and non-spontaneous at high temperatures, what must be true about the change of enthalphy (

Tap to reveal answer
A reaction is spontaneous if the change of Gibb's free energy ( G) is less than zero. Recall that (
G) is less than zero. Recall that ( G) is related to
G) is related to  H and
H and  S by the equation below.
S by the equation below.

For this particular reaction,  S is negative because the total number of moles of gas decreases from reactants to products. Since the reaction is spontaneous at lower temperatures, then
S is negative because the total number of moles of gas decreases from reactants to products. Since the reaction is spontaneous at lower temperatures, then  G must be negative when T is small. Since the -T
G must be negative when T is small. Since the -T S term would be positive for all values of T, the only way
S term would be positive for all values of T, the only way  G can be negative is if
G can be negative is if  H is negative. At higher temperatures, the positve -T
H is negative. At higher temperatures, the positve -T S term would outweigh the negative
S term would outweigh the negative  H term, resulting in a positive
H term, resulting in a positive  G and a non-spontaneous reaction.
G and a non-spontaneous reaction.
A reaction is spontaneous if the change of Gibb's free energy (



For this particular reaction, 







← Didn't Know|Knew It →
A system with and will never be spontaneous.
A system with and will never be spontaneous.
Tap to reveal answer
The Gibb's free energy equation is used to determine the spontaneity of a reaction and is written as follows:  .
.
 is Gibb's free energy,
is Gibb's free energy,  is enthalpy, and
is enthalpy, and  is entropy. In order for a reaction to be spontaneous, Gibb's free energy must have a negative value. Based on the equation, we can see that a positive enthalpy in combination with a negative entropy will always result in a positive value for Gibb's free energy.
is entropy. In order for a reaction to be spontaneous, Gibb's free energy must have a negative value. Based on the equation, we can see that a positive enthalpy in combination with a negative entropy will always result in a positive value for Gibb's free energy.


This means these are the conditions that will always result in a nonspontaneous reaction.
The Gibb's free energy equation is used to determine the spontaneity of a reaction and is written as follows: 



This means these are the conditions that will always result in a nonspontaneous reaction.
← Didn't Know|Knew It →
A chemical reaction has the following changes in enthalpy and entropy.


What is the temperature range for this reaction that allow it to be spontaneous?
A chemical reaction has the following changes in enthalpy and entropy.
What is the temperature range for this reaction that allow it to be spontaneous?
Tap to reveal answer
A reaction is spontaneous when Gibb's free energy is negative. As a result, we need to determine the temperature range where Gibb's free energy is less than zero. Since we know the values for changes in enthalpy and entropy, we can plug them into the Gibb's free energy equation, and set it equal to zero.




437K is the temperature at which Gibb's free energy is zero. Since entropy is positive for this reaction, increasing the temperature will result in a more negative value for Gibb's free energy.

As a result, any temperature that is greater than 437K will make this reaction spontaneous.
A reaction is spontaneous when Gibb's free energy is negative. As a result, we need to determine the temperature range where Gibb's free energy is less than zero. Since we know the values for changes in enthalpy and entropy, we can plug them into the Gibb's free energy equation, and set it equal to zero.
437K is the temperature at which Gibb's free energy is zero. Since entropy is positive for this reaction, increasing the temperature will result in a more negative value for Gibb's free energy.
As a result, any temperature that is greater than 437K will make this reaction spontaneous.
← Didn't Know|Knew It →
Consider the following reaction in a galvanic cell:

Which if the following statements about the reaction is false?
Consider the following reaction in a galvanic cell:
Which if the following statements about the reaction is false?
Tap to reveal answer
A galvanic cell results in a positive cell potential from a spontaneous reaction. Spontaneous reactions always have a negative Gibb's free energy.
An equilibrium constant greater than one would indicate that the equilibrium concentration of products is greater than the equilibrium concentration of reactants, consistent with a spontaneous reaction.
A galvanic cell results in a positive cell potential from a spontaneous reaction. Spontaneous reactions always have a negative Gibb's free energy.
An equilibrium constant greater than one would indicate that the equilibrium concentration of products is greater than the equilibrium concentration of reactants, consistent with a spontaneous reaction.
← Didn't Know|Knew It →
Consider the following reaction in a galvanic cell:
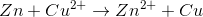
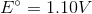
What is the Gibb's free energy for the reaction under standard conditions?

Consider the following reaction in a galvanic cell:
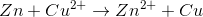
What is the Gibb's free energy for the reaction under standard conditions?
Tap to reveal answer
You can find the Gibb's free energy of a galvanic cell by using the following equation:

 is the number of moles of electrons that are transferred in the reaction,
is the number of moles of electrons that are transferred in the reaction,  is Faraday's constant, and
is Faraday's constant, and  is the potential of the cell.
is the potential of the cell.
We are given the constant value and the cell potential. The moles of electrons transferred is equal to the change in charge on the atoms in the reaction. In this reaction, copper is reduced from a charge of  to zero, requiring that is gained two moles of electrons.
to zero, requiring that is gained two moles of electrons.
Using these values, we can find Gibb's free energy for the cell.


Notice that the value is negative. Galvanic cells are spontaneous, and will have negative Gibb's free energies at standard conditions.
You can find the Gibb's free energy of a galvanic cell by using the following equation:



We are given the constant value and the cell potential. The moles of electrons transferred is equal to the change in charge on the atoms in the reaction. In this reaction, copper is reduced from a charge of 
Using these values, we can find Gibb's free energy for the cell.
Notice that the value is negative. Galvanic cells are spontaneous, and will have negative Gibb's free energies at standard conditions.
← Didn't Know|Knew It →
Suppose that a reaction with an equilibrium constant equal to  occurs while at standard state conditions. Which of the following is true regarding this reaction?
occurs while at standard state conditions. Which of the following is true regarding this reaction?
Suppose that a reaction with an equilibrium constant equal to 
Tap to reveal answer
For this question, we're told that a reaction is being run under standard conditions, and that the equilibrium constant for this reaction is much greater than 1. With this information in mind, we can find the correct answer without even having to resort to math. Since the equilibrium constant is greater than one, we know that the products of this reaction are favored over the reactants. And since the products are favored, this means that the reaction must be shifted to the right, in which case it is spontaneous. It's important to know that a spontaneous reaction will always have a negative change in Gibb's energy.
For completion's sake, however, we can show that the Gibb's free energy change is negative by utilizing the free energy change equation.

From this equation, we can see that if  is greater than one (as it is in the reaction for this question) the natural logarithm of this value will also be positive. And since the ideal gas constant and the absolute temperature are also positive values, the product of all these values will be positive. But, there is a negative sign in front of these terms, hence making our final answer negative.
is greater than one (as it is in the reaction for this question) the natural logarithm of this value will also be positive. And since the ideal gas constant and the absolute temperature are also positive values, the product of all these values will be positive. But, there is a negative sign in front of these terms, hence making our final answer negative.
For this question, we're told that a reaction is being run under standard conditions, and that the equilibrium constant for this reaction is much greater than 1. With this information in mind, we can find the correct answer without even having to resort to math. Since the equilibrium constant is greater than one, we know that the products of this reaction are favored over the reactants. And since the products are favored, this means that the reaction must be shifted to the right, in which case it is spontaneous. It's important to know that a spontaneous reaction will always have a negative change in Gibb's energy.
For completion's sake, however, we can show that the Gibb's free energy change is negative by utilizing the free energy change equation.
From this equation, we can see that if 
← Didn't Know|Knew It →
Which of the following situations describes a reaction that can never be spontaneous?
Which of the following situations describes a reaction that can never be spontaneous?
Tap to reveal answer
In this question, we're being asked to provide a circumstance that will never result in a spontaneous reaction. In other words, which answer choice presents a situation that will always be non-spontaneous.
To evaluate the spontaneity of a reaction, it's essential to look at the change in the Gibb's free energy of that reaction. A negative change results in a spontaneous reaction, whereas a positive change results in a non-spontaneous reaction.
We can see that each of the answer choices mentions enthalpy and entropy. Therefore, we need to be able to relate these two terms with the Gibb's free energy term. To do this, we can make use of the following expression.

Again, in order to have a non-spontaneous reaction, the  term shown above needs to have a positive value.
term shown above needs to have a positive value.

From the above expression, we can conclude that any process that has a positive  and a negative
and a negative  will always be positive, and hence will always result in a non-spontaneous reaction.
will always be positive, and hence will always result in a non-spontaneous reaction.
In this question, we're being asked to provide a circumstance that will never result in a spontaneous reaction. In other words, which answer choice presents a situation that will always be non-spontaneous.
To evaluate the spontaneity of a reaction, it's essential to look at the change in the Gibb's free energy of that reaction. A negative change results in a spontaneous reaction, whereas a positive change results in a non-spontaneous reaction.
We can see that each of the answer choices mentions enthalpy and entropy. Therefore, we need to be able to relate these two terms with the Gibb's free energy term. To do this, we can make use of the following expression.
Again, in order to have a non-spontaneous reaction, the 
From the above expression, we can conclude that any process that has a positive 

← Didn't Know|Knew It →
If the reaction quotient (Q) is greater than the equilibrium constant (K), what is true about the Gibbs free energy?
If the reaction quotient (Q) is greater than the equilibrium constant (K), what is true about the Gibbs free energy?
Tap to reveal answer
If Q is greater than K, the reaction has exceeded the equilibrium state. It will proceed nonspontaneously (since equilibrium has already been reached), and this must mean that the ΔG (gibbs free energy) must be positive, or greater than zero.
If Q is greater than K, the reaction has exceeded the equilibrium state. It will proceed nonspontaneously (since equilibrium has already been reached), and this must mean that the ΔG (gibbs free energy) must be positive, or greater than zero.
← Didn't Know|Knew It →
Of the following reactions, which of the following is only spontaneous at high enough temperatures?
Of the following reactions, which of the following is only spontaneous at high enough temperatures?
Tap to reveal answer
Based on Gibbs Free Energy, ∆G = ∆H – T∆S, we find that ∆H– will contribute to spontaneity (∆G<0). Since we are looking at temperatures, in order for ∆S to make an nonspontaneous reaction spontaneous at only high temperatures, ∆H will be positive, and ∆S will be positive.
Based on Gibbs Free Energy, ∆G = ∆H – T∆S, we find that ∆H– will contribute to spontaneity (∆G<0). Since we are looking at temperatures, in order for ∆S to make an nonspontaneous reaction spontaneous at only high temperatures, ∆H will be positive, and ∆S will be positive.
← Didn't Know|Knew It →
Suppose that a rxn has ∆H = -28 kJ and ∆S= -60 J/K. At what temperature will it
change from spontaneous to non-spontaneous?
Suppose that a rxn has ∆H = -28 kJ and ∆S= -60 J/K. At what temperature will it
change from spontaneous to non-spontaneous?
Tap to reveal answer
Approximately 467 K. ∆G=∆H-T∆S and a rxn proceeds spontaneously when ∆G < 0
and is non-spontaneous when ∆G > 0. So if we set ∆G=0 and solve the equation for T, we will see that the crossover from spontaneous to non-spontaneous occurs when T=467K.
Approximately 467 K. ∆G=∆H-T∆S and a rxn proceeds spontaneously when ∆G < 0
and is non-spontaneous when ∆G > 0. So if we set ∆G=0 and solve the equation for T, we will see that the crossover from spontaneous to non-spontaneous occurs when T=467K.
← Didn't Know|Knew It →
If a reaction has a positive value for its enthalpy and a negative value for its entropy, which of the following is true?
If a reaction has a positive value for its enthalpy and a negative value for its entropy, which of the following is true?
Tap to reveal answer
Given the equation for free energy, (delta)G = (delta)H-T(delta)S, we can determine that the reaction is nonspontaneous at all temperatures if H is positive and S is negative. This combination would always lead to a positive G value, meaning that free energy is required for the reaction to take place and it is therefore nonspontaneous.
Given the equation for free energy, (delta)G = (delta)H-T(delta)S, we can determine that the reaction is nonspontaneous at all temperatures if H is positive and S is negative. This combination would always lead to a positive G value, meaning that free energy is required for the reaction to take place and it is therefore nonspontaneous.
← Didn't Know|Knew It →