Chemical Reactions - AP Chemistry
Card 1 of 247
What is the chemical formula of the salt formed when a chemist mixes solvated Potassium and Arsenic ions in solution?
What is the chemical formula of the salt formed when a chemist mixes solvated Potassium and Arsenic ions in solution?
Tap to reveal answer
Potassium is a Group I element, so to get to a filled valence shell, it will lost one electron, yielding  .
.
Arsenic is a Group 5 element, so it needs to gain three electrons to obtain a filled valence shell, yielding  .
.
In order to balance out the charges, the resultant salt will be  .
.
Potassium is a Group I element, so to get to a filled valence shell, it will lost one electron, yielding 
Arsenic is a Group 5 element, so it needs to gain three electrons to obtain a filled valence shell, yielding 
In order to balance out the charges, the resultant salt will be 
← Didn't Know|Knew It →
What is the net ionic equation for the ion exchange reaction between ferrous sulfate and calcium iodide? Assume all compounds are soluble.
What is the net ionic equation for the ion exchange reaction between ferrous sulfate and calcium iodide? Assume all compounds are soluble.
Tap to reveal answer
First, we must know what ferrous sulfate is. Ferrous refers to  , and sulfate has the formula
, and sulfate has the formula  . When we combine the two together we get
. When we combine the two together we get  .
.
Calcium is a divatent cation and iodide is a monovalent anion, so their salt is  . The ion exchange reaction is then:
. The ion exchange reaction is then:

First, we must know what ferrous sulfate is. Ferrous refers to 


Calcium is a divatent cation and iodide is a monovalent anion, so their salt is 
← Didn't Know|Knew It →
Select the net ionic equation from this molecular reaction:

Select the net ionic equation from this molecular reaction:
Tap to reveal answer
The net ionic equation is derived by removing all spectator ions from the total ionic equation (in which all ions are listed). To put it another way, the net ionic equation involves only the ions that participate in a reaction which, in this case, is the precipitation of barium sulfate.
Begin by writing all aqueous compounds in their dissociated (ionic) forms.


Cancel out any ions that appear in equal quantities on both sides of the equation. In this case, we can cancel the nitrate and potassium ions.

This is our net ionic equation.
The net ionic equation is derived by removing all spectator ions from the total ionic equation (in which all ions are listed). To put it another way, the net ionic equation involves only the ions that participate in a reaction which, in this case, is the precipitation of barium sulfate.
Begin by writing all aqueous compounds in their dissociated (ionic) forms.
Cancel out any ions that appear in equal quantities on both sides of the equation. In this case, we can cancel the nitrate and potassium ions.
This is our net ionic equation.
← Didn't Know|Knew It →

The reaction above could be classified as .
The reaction above could be classified as .
Tap to reveal answer
When one molecule is converted into two or more smaller molecules, the reaction is considered a decomposition reaction. In this example, none of the elements under go a change in oxidation number, so this is not an oxidation-reduction reaction. (Calcium is always  , oxygen is always
, oxygen is always  , and carbon remains
, and carbon remains  throughout the reaction.)
throughout the reaction.)
When one molecule is converted into two or more smaller molecules, the reaction is considered a decomposition reaction. In this example, none of the elements under go a change in oxidation number, so this is not an oxidation-reduction reaction. (Calcium is always 


← Didn't Know|Knew It →
What is the balanced chemical equation for the combustion of butane  ?
?
What is the balanced chemical equation for the combustion of butane 
Tap to reveal answer
Combustion is the chemical reaction of a hydrocarbon with molecular oxygen, and it always produces carbon dioxide and water. Knowing the reactants and products, the unbalanced equation must be:

We start by balancing the hydrogens. Since there are 10 on the left and only 2 on the right, we put a coefficient of 5 on water.

Similarly, we balance carbons by putting a 4 on the carbon dioxide.

To find the number of oxygens on the right, we multiply the 4 coefficient by the 2 subscript on O (which gets us 8 oxygens) and then add the 5 oxygens from the 5 water molecules to get a total of 13. The needed coefficient for  on the left would then have to be 13/2.
on the left would then have to be 13/2.

Because fractional coefficients are not allowed, we mutiply every coefficient by 2 to find our final reaction:


Combustion is the chemical reaction of a hydrocarbon with molecular oxygen, and it always produces carbon dioxide and water. Knowing the reactants and products, the unbalanced equation must be:
We start by balancing the hydrogens. Since there are 10 on the left and only 2 on the right, we put a coefficient of 5 on water.
Similarly, we balance carbons by putting a 4 on the carbon dioxide.
To find the number of oxygens on the right, we multiply the 4 coefficient by the 2 subscript on O (which gets us 8 oxygens) and then add the 5 oxygens from the 5 water molecules to get a total of 13. The needed coefficient for 
Because fractional coefficients are not allowed, we mutiply every coefficient by 2 to find our final reaction:
← Didn't Know|Knew It →
Determine whether or not solid aluminum reacts with aqueous zinc chloride. If it does, determine the balanced equation for the reaction.
Determine whether or not solid aluminum reacts with aqueous zinc chloride. If it does, determine the balanced equation for the reaction.
Tap to reveal answer
When we check the activity series, it is fairly easy to see that aluminum metal is more reactive than zinc metal. So, in this case, the two metals undergo a redox reaction, where the aqueous  is reduced to solid
is reduced to solid  , and the solid
, and the solid  is oxidized to aqueous
is oxidized to aqueous  . These charges are the common oxidation states for zinc and aluminum and should be memorized.
. These charges are the common oxidation states for zinc and aluminum and should be memorized.
Because  is the new species, it bonds with 3
is the new species, it bonds with 3  ions. The unbalanced equation is:
ions. The unbalanced equation is:

We note that there are 2 chlorine atoms on the left and 3 chlorine atoms on the right. To balance, we use a 3 coefficient on the left and a 2 coefficient on the right. This gives a total of 6 chlorine atoms on eahc side.

However, now we have also increased the amounts of zinc and aluminum. We copy the necessary coefficients to balance those—2 for aluminum on the left, 3 for zinc on the right—and we are done:

When we check the activity series, it is fairly easy to see that aluminum metal is more reactive than zinc metal. So, in this case, the two metals undergo a redox reaction, where the aqueous 



Because 

We note that there are 2 chlorine atoms on the left and 3 chlorine atoms on the right. To balance, we use a 3 coefficient on the left and a 2 coefficient on the right. This gives a total of 6 chlorine atoms on eahc side.
However, now we have also increased the amounts of zinc and aluminum. We copy the necessary coefficients to balance those—2 for aluminum on the left, 3 for zinc on the right—and we are done:
← Didn't Know|Knew It →

What is the net ionic form of this equation?
What is the net ionic form of this equation?
Tap to reveal answer
To find the net ionic form of this chemical equation, first balance the equation, then dissociate all soluble components. In other words, separate the aqueous terms into two separate molecules/atoms. For example,  dissociates into
dissociates into  and
and  . After separating all soluble terms, balance the equation and cancel out any terms that repeat on both sides of the equation. For example,
. After separating all soluble terms, balance the equation and cancel out any terms that repeat on both sides of the equation. For example,  will be on both sides of the equation after separating all terms so you can cross them out. Below are the steps taken to find the net ionic form of this chemical equation.
will be on both sides of the equation after separating all terms so you can cross them out. Below are the steps taken to find the net ionic form of this chemical equation.

Write out the dissociated forms of each species, if applicable.

Cross out species that appear on both sides of the equation. The net ionic equation is:

To find the net ionic form of this chemical equation, first balance the equation, then dissociate all soluble components. In other words, separate the aqueous terms into two separate molecules/atoms. For example, 



Write out the dissociated forms of each species, if applicable.
Cross out species that appear on both sides of the equation. The net ionic equation is:
← Didn't Know|Knew It →

What type of reaction is shown above?
What type of reaction is shown above?
Tap to reveal answer
The typical double replacement reaction is as follows:


The anions are the chemical species being exchanged (both sulfur and chloride). Hence, the name double replacement is given to this particular reaction.
The typical double replacement reaction is as follows:
The anions are the chemical species being exchanged (both sulfur and chloride). Hence, the name double replacement is given to this particular reaction.
← Didn't Know|Knew It →
Which of the following represents a combustion reaction?
Which of the following represents a combustion reaction?
Tap to reveal answer
Combustion reactions always produce 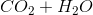 and excess heat. Depending on the molecule that is oxidized by oxygen, which in this case was methane
and excess heat. Depending on the molecule that is oxidized by oxygen, which in this case was methane 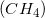 , the ratios between
, the ratios between  and
and 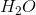 can vary.
can vary.
Combustion reactions always produce 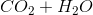
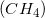

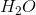
← Didn't Know|Knew It →
Given that lithium is higher on the activity series than hydrogen, what should be seen when solid lithium and hydrochloric acid are mixed?
Given that lithium is higher on the activity series than hydrogen, what should be seen when solid lithium and hydrochloric acid are mixed?
Tap to reveal answer
Given that lithium is more reactive than hydrogen, expect a single replacement reaction to take place, so lithium will replace hydrogen.

All ionic compounds are solids at room temperature, which is why lithium chloride has the solid phase label. Remember that hydrogen is a diatomic element which is a gas a room temperature. When observing reactions in which gasses are produced from a solution, bubbles are seen. There is no indication whether this reaction is endothermic or exothermic, nor is there any information that suggests the reaction should change color.
Given that lithium is more reactive than hydrogen, expect a single replacement reaction to take place, so lithium will replace hydrogen.
All ionic compounds are solids at room temperature, which is why lithium chloride has the solid phase label. Remember that hydrogen is a diatomic element which is a gas a room temperature. When observing reactions in which gasses are produced from a solution, bubbles are seen. There is no indication whether this reaction is endothermic or exothermic, nor is there any information that suggests the reaction should change color.
← Didn't Know|Knew It →
The following unbalanced reaction is important in the process of Steel Manufacturing:

Balance the following equation and name it's reaction type:

The following unbalanced reaction is important in the process of Steel Manufacturing:
Balance the following equation and name it's reaction type:
Tap to reveal answer
Use the following steps to balance the equation:
1.) Check for diatomic molecules: in this case there are none so move on to step 2
2.) Balance the metals (hydrogen is not considered a metal in this application): There are 2 iron atoms on the left hand side and 1 iron atom on the right hand side so we add a 2 in front of the Fe on the right hand side to give:

3.) Balance the nonmetals (not including oxygen). There is one carbon on both sides so move on to step 4
4.) Balance oxygen. There are 3 oxygens on the left hand side and only 1 on the right hand side, so we can add a 3 in front of carbon monoxide on the right hand side to give:

5.) Balance hydrogen. No hydrogens, so we move on.
6.) Recount all atoms to make sure you have balanced correctly. There are 2 iron atoms on both sides and 3 oxygen on both sides. However our carbons are no longer balanced so we can add a 3 in front of the elemental carbon on the left hand side to give:

If this problem had involved ionic species you would also want to make certain that the charges are balanced on both sides of the equation.
The charges on both sides add up to zero so your equation is balanced:

Single v.s Double Replacement Reaction:
Single replacement Reaction:
A single replacement reaction is defined as a type of reduction-oxidation reaction in which a more active element replaces a less active element in a compound. These reactions produce a new compound and an element as the products.
General chemical equation:

Double Replacement Reaction:
A double replacement reaction is a type of reaction in which two reacting chemical species exchange bonds (ionic or covalent) resulting in the creation of two new products.
General chemical equation:

 is also important in steel manufacturing and is a single replacement reaction. However, the equation given was
is also important in steel manufacturing and is a single replacement reaction. However, the equation given was so the difference being carbon monoxide production rather than carbon dioxide production. In reality both reactions occur simultaneously.
so the difference being carbon monoxide production rather than carbon dioxide production. In reality both reactions occur simultaneously.
Use the following steps to balance the equation:
1.) Check for diatomic molecules: in this case there are none so move on to step 2
2.) Balance the metals (hydrogen is not considered a metal in this application): There are 2 iron atoms on the left hand side and 1 iron atom on the right hand side so we add a 2 in front of the Fe on the right hand side to give:
3.) Balance the nonmetals (not including oxygen). There is one carbon on both sides so move on to step 4
4.) Balance oxygen. There are 3 oxygens on the left hand side and only 1 on the right hand side, so we can add a 3 in front of carbon monoxide on the right hand side to give:
5.) Balance hydrogen. No hydrogens, so we move on.
6.) Recount all atoms to make sure you have balanced correctly. There are 2 iron atoms on both sides and 3 oxygen on both sides. However our carbons are no longer balanced so we can add a 3 in front of the elemental carbon on the left hand side to give:
If this problem had involved ionic species you would also want to make certain that the charges are balanced on both sides of the equation.
The charges on both sides add up to zero so your equation is balanced:
Single v.s Double Replacement Reaction:
Single replacement Reaction:
A single replacement reaction is defined as a type of reduction-oxidation reaction in which a more active element replaces a less active element in a compound. These reactions produce a new compound and an element as the products.
General chemical equation:
Double Replacement Reaction:
A double replacement reaction is a type of reaction in which two reacting chemical species exchange bonds (ionic or covalent) resulting in the creation of two new products.
General chemical equation:


← Didn't Know|Knew It →
What is used as guidelines to determine the phase labels for double displacement/double replacement reactions?
What is used as guidelines to determine the phase labels for double displacement/double replacement reactions?
Tap to reveal answer
For double displacement, the solubility rules determine the phase labels. Make sure the correct set of rules/guidelines for the type of reaction that is being written/balanced are used. The octet rule refers to the stability atoms achieve by filling their outer shell of electrons. Huckel number is 4n+2 where n is any integer, and is involved in assigning aromaticity.
For double displacement, the solubility rules determine the phase labels. Make sure the correct set of rules/guidelines for the type of reaction that is being written/balanced are used. The octet rule refers to the stability atoms achieve by filling their outer shell of electrons. Huckel number is 4n+2 where n is any integer, and is involved in assigning aromaticity.
← Didn't Know|Knew It →
For single replacement reactions, which of the following sets of guidelines are most helpful?
For single replacement reactions, which of the following sets of guidelines are most helpful?
Tap to reveal answer
When writing/balancing a single replacement reaction, make sure to consult the activity series, because that will tell us if a reaction will actually take place. Remember to look at the cations (metals and hydrogen) and where they are in relation to each other on the activity series. The higher one on the activity series is the most reactive; so if the cation by itself is the most reactive, it will replace the other cation and a reaction will occur. Boyle's law explains the inverse relationship between volume and pressure with respect to certain amount of an ideal gas at constant temperature.
When writing/balancing a single replacement reaction, make sure to consult the activity series, because that will tell us if a reaction will actually take place. Remember to look at the cations (metals and hydrogen) and where they are in relation to each other on the activity series. The higher one on the activity series is the most reactive; so if the cation by itself is the most reactive, it will replace the other cation and a reaction will occur. Boyle's law explains the inverse relationship between volume and pressure with respect to certain amount of an ideal gas at constant temperature.
← Didn't Know|Knew It →

What is the coefficient for  , reduced to the lowest whole number, when the given reaction is balanced?
, reduced to the lowest whole number, when the given reaction is balanced?
What is the coefficient for 
Tap to reveal answer
The best way to solve this problem is to try all the possible answers. When we try 8,  means that there must be
means that there must be  .
.  means that there must be just
means that there must be just  , which means that there is
, which means that there is  . When
. When  is reduced to
is reduced to  , there are 5 remaining electrons. So 8 is correct. Alternatively, this question may be answered by the classical "trial and error" method of balancing equations.
, there are 5 remaining electrons. So 8 is correct. Alternatively, this question may be answered by the classical "trial and error" method of balancing equations.
The best way to solve this problem is to try all the possible answers. When we try 8, 






← Didn't Know|Knew It →
What is the net ionic equation for the reaction that occurs when sodium chloride is mixed with silver nitrate.
What is the net ionic equation for the reaction that occurs when sodium chloride is mixed with silver nitrate.
Tap to reveal answer
Only the Ag and Cl ions are reacting to form solid silver chloride.
Only the Ag and Cl ions are reacting to form solid silver chloride.
← Didn't Know|Knew It →
What is the net ionic equation that occurs when sodium chloride is mixed with potassium nitrate?
What is the net ionic equation that occurs when sodium chloride is mixed with potassium nitrate?
Tap to reveal answer
Since no precipitation is predicted by the solubility rules, there will be no reaction.
Since no precipitation is predicted by the solubility rules, there will be no reaction.
← Didn't Know|Knew It →
What is the net ionic reaction when potassium carbonate is mixed with Calcium bromide?
What is the net ionic reaction when potassium carbonate is mixed with Calcium bromide?
Tap to reveal answer
Only the calcium and carbonate ions are reacting according to the solubility rules to form calcium carbonate.
Only the calcium and carbonate ions are reacting according to the solubility rules to form calcium carbonate.
← Didn't Know|Knew It →
What is the net ionic reaction when a solution of Cd(NO3)2 is reacted with (NH4)2S?
What is the net ionic reaction when a solution of Cd(NO3)2 is reacted with (NH4)2S?
Tap to reveal answer
Only Cd and S ions are interacting according to the solubility rules to form a solid precipitant.
Only Cd and S ions are interacting according to the solubility rules to form a solid precipitant.
← Didn't Know|Knew It →
What is the net ionic reaction when a solution of rubidium sulfate is mixed with barium hydroxide?
What is the net ionic reaction when a solution of rubidium sulfate is mixed with barium hydroxide?
Tap to reveal answer
Only the Ba and sulfate ions will react according to the solubility rules.
Only the Ba and sulfate ions will react according to the solubility rules.
← Didn't Know|Knew It →
What is the chemical formula of the salt formed when a chemist mixes solvated Potassium and Arsenic ions in solution?
What is the chemical formula of the salt formed when a chemist mixes solvated Potassium and Arsenic ions in solution?
Tap to reveal answer
Potassium is a Group I element, so to get to a filled valence shell, it will lost one electron, yielding  .
.
Arsenic is a Group 5 element, so it needs to gain three electrons to obtain a filled valence shell, yielding  .
.
In order to balance out the charges, the resultant salt will be  .
.
Potassium is a Group I element, so to get to a filled valence shell, it will lost one electron, yielding 
Arsenic is a Group 5 element, so it needs to gain three electrons to obtain a filled valence shell, yielding 
In order to balance out the charges, the resultant salt will be 
← Didn't Know|Knew It →