Balancing Equations - AP Chemistry
Card 1 of 172
When the equation for the reaction shown below is balanced with the lowest whole-number coefficients, what is the sum of the coefficients of the reactants?
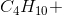



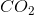
When the equation for the reaction shown below is balanced with the lowest whole-number coefficients, what is the sum of the coefficients of the reactants?
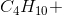



Tap to reveal answer
The coefficients of the equation, when balanced, should be 2, 13, 10, and 8. This problem wants to know the sum of the reactants, so only the coefficients on the left side of the reactants should be added. The sum of 2 and 13 is 15, so 15 is the correct answer.
The coefficients of the equation, when balanced, should be 2, 13, 10, and 8. This problem wants to know the sum of the reactants, so only the coefficients on the left side of the reactants should be added. The sum of 2 and 13 is 15, so 15 is the correct answer.
← Didn't Know|Knew It →
Balance the following equation:
FeCl3 + NOH5 → Fe(OH)3 + NH4Cl
Balance the following equation:
FeCl3 + NOH5 → Fe(OH)3 + NH4Cl
Tap to reveal answer
The first thing to do is to balance the Cl (3 on the left; one on the right) ← add 3 for NH4Cl
Now, there are 3 N on the right and only one on the left; add a 3 to the NOH5
Check to see that the Fe, H, and O balance, which they do
The first thing to do is to balance the Cl (3 on the left; one on the right) ← add 3 for NH4Cl
Now, there are 3 N on the right and only one on the left; add a 3 to the NOH5
Check to see that the Fe, H, and O balance, which they do
← Didn't Know|Knew It →
Balance the following equation:
NaOH + H2SO4 → H2O + Na2SO4
Balance the following equation:
NaOH + H2SO4 → H2O + Na2SO4
Tap to reveal answer
Balancing equations:
there is 1 NaOH and 1 H2SO4 in the reactants; and 1 H2O and 1 Na2SO4 in the products
steps:
-
balance the Na first; get 2 NaOH
-
balance H next; get 1 H2SO4, 2 H2O
-
check to make sure that O and S balance
Balancing equations:
there is 1 NaOH and 1 H2SO4 in the reactants; and 1 H2O and 1 Na2SO4 in the products
steps:
-
balance the Na first; get 2 NaOH
-
balance H next; get 1 H2SO4, 2 H2O
-
check to make sure that O and S balance
← Didn't Know|Knew It →
_Fe2O3 + _HCl ⇌ _FeCl3 + _H2O
The Following question will be based on the unbalanced reaction above
For the reaction above to be balanced what coefficient should be in front of the compound HCl?
_Fe2O3 + _HCl ⇌ _FeCl3 + _H2O
The Following question will be based on the unbalanced reaction above
For the reaction above to be balanced what coefficient should be in front of the compound HCl?
Tap to reveal answer
Balancing reactions is best acheived by using a stepwise approach. It is useful to work through each atom making sure it is present in a balanced fashion on both sides of the equation. Starting with Fe, Fe2O3 is the only molecule with Fe present on the left side of the equation and FeCl3 is the only molecule with Fe present on the right side of the equation. Thus the molar ratio of Fe2O3 to FeCl3 must be 1:2. Moving on to O, the O on the left side of the equation exists as Fe2O3 and H2O on the right. The molar ratio of Fe2O3 to H2O is therefore 1:3. Cl exists in HCl on the left and FeCl3 on the right. Thus the molar ratio of HCl to FeCl3 must be 3:1. To ensure that these molar ratios are maintaned the balanced formula is then determined to be Fe2O3 + 6HCl -> 2FeCl3 + 3H2O
Balancing reactions is best acheived by using a stepwise approach. It is useful to work through each atom making sure it is present in a balanced fashion on both sides of the equation. Starting with Fe, Fe2O3 is the only molecule with Fe present on the left side of the equation and FeCl3 is the only molecule with Fe present on the right side of the equation. Thus the molar ratio of Fe2O3 to FeCl3 must be 1:2. Moving on to O, the O on the left side of the equation exists as Fe2O3 and H2O on the right. The molar ratio of Fe2O3 to H2O is therefore 1:3. Cl exists in HCl on the left and FeCl3 on the right. Thus the molar ratio of HCl to FeCl3 must be 3:1. To ensure that these molar ratios are maintaned the balanced formula is then determined to be Fe2O3 + 6HCl -> 2FeCl3 + 3H2O
← Didn't Know|Knew It →
After the following reaction is balanced, how many moles of H2O can 4 moles of C2H6 produce?
__C2H6(s) + O2(g) → H2O(l) + CO2(g)
After the following reaction is balanced, how many moles of H2O can 4 moles of C2H6 produce?
__C2H6(s) + O2(g) → H2O(l) + CO2(g)
Tap to reveal answer
The reaction balances out to 2C2H6(s) + 7O2(g) → 6H2O(l) + 4CO2(g). For every 2 moles of C2H6 you can produce 6 moles of H2O. Giving you a total production of 12 moles when you have 4 moles of C2H6.
The reaction balances out to 2C2H6(s) + 7O2(g) → 6H2O(l) + 4CO2(g). For every 2 moles of C2H6 you can produce 6 moles of H2O. Giving you a total production of 12 moles when you have 4 moles of C2H6.
← Didn't Know|Knew It →
Consider the following unbalanced equation for the combustion of propane,  :
:

If you were to combust one mole of propane, how many moles of water would you produce?
Consider the following unbalanced equation for the combustion of propane, 
If you were to combust one mole of propane, how many moles of water would you produce?
Tap to reveal answer
Begin by balancing the equation. There are many ways to do this, but one method that is particularly useful is to assume that you have 1 mole of your hydrocarbon (propane), and balance the equation from there. It may be necessary to manipulate an equation further if you end up with fractions, but all you will need to do is multiply by an integer if that is the case.
First, we will balance the carbons. There are three carbons in propane, so we will make sure there are three carbons on the right side of the arrow as well:

This is not complete. We will next balance the hydrogens. There are eight hydrogens on the left side of the equation, so:

The last step is to balance the oxygens on the left and right side of the equation

Our equation is balanced and all coefficients are integers. If we begin with one mole of propane, we will produce four moles of water.
Begin by balancing the equation. There are many ways to do this, but one method that is particularly useful is to assume that you have 1 mole of your hydrocarbon (propane), and balance the equation from there. It may be necessary to manipulate an equation further if you end up with fractions, but all you will need to do is multiply by an integer if that is the case.
First, we will balance the carbons. There are three carbons in propane, so we will make sure there are three carbons on the right side of the arrow as well:
This is not complete. We will next balance the hydrogens. There are eight hydrogens on the left side of the equation, so:
The last step is to balance the oxygens on the left and right side of the equation
Our equation is balanced and all coefficients are integers. If we begin with one mole of propane, we will produce four moles of water.
← Didn't Know|Knew It →
Consider the following unbalanced equation:

How many grams of solid iron are needed to make 36.0g of  ? Assume that chlorine is in excess.
? Assume that chlorine is in excess.
Consider the following unbalanced equation:
How many grams of solid iron are needed to make 36.0g of 
Tap to reveal answer

First, we will balance the equation:

Since chlorine is in excess, we know that the limiting reagent is iron.

First, we will balance the equation:
Since chlorine is in excess, we know that the limiting reagent is iron.
← Didn't Know|Knew It →
Consider the following reaction:

When the equation is balanced, what will be the coefficient in front of HCl?
Consider the following reaction:
When the equation is balanced, what will be the coefficient in front of HCl?
Tap to reveal answer
When balancing equations, the goal is to make sure that the same atoms, in both type and amount, are on both the reactant and product side of the equation. A helpful approach is to write down the number of atoms already on both sides of the unbalanced equation. This way, you can predict which compounds need to be increased on which side in order to balance the equation. It also helps to balance oxygen and hydrogen last in the equation.
In this reaction, we can balance as follows.
Reactants: 1K, 1Mn, 1Cl, 4O, 1H
Products: 1K, 1Mn, 5Cl, 1O, 2H
So, we will need to increase H2O and HCl. The final balanced equation is written below.

When balancing equations, the goal is to make sure that the same atoms, in both type and amount, are on both the reactant and product side of the equation. A helpful approach is to write down the number of atoms already on both sides of the unbalanced equation. This way, you can predict which compounds need to be increased on which side in order to balance the equation. It also helps to balance oxygen and hydrogen last in the equation.
In this reaction, we can balance as follows.
Reactants: 1K, 1Mn, 1Cl, 4O, 1H
Products: 1K, 1Mn, 5Cl, 1O, 2H
So, we will need to increase H2O and HCl. The final balanced equation is written below.
← Didn't Know|Knew It →
Balance the following chemical equation.

Balance the following chemical equation.
Tap to reveal answer
To balance an equation, we need to make sure there is the same amount of elements to the left of the arrow as there is to the right. We also need all the charges to balance out. We notice right away that there are three chlorine atoms on the left, but only one on the right.
 (1Na, 1O, 1H, 1Fe, 3Cl : 1Na, 3O, 3H, 1Fe, 1Cl)
(1Na, 1O, 1H, 1Fe, 3Cl : 1Na, 3O, 3H, 1Fe, 1Cl)
We can solve this by multiplying NaCl by three.
 (1Na, 1O, 1H, 1Fe, 3Cl : 3Na, 3O, 3H, 1Fe, 3Cl)
(1Na, 1O, 1H, 1Fe, 3Cl : 3Na, 3O, 3H, 1Fe, 3Cl)
This causes us to have an imbalance of sodium, which we can correct by manipulating NaOH.
 (3Na, 3O, 3H, 1Fe, 3Cl : 3Na, 3O, 3H, 1Fe, 1Cl)
(3Na, 3O, 3H, 1Fe, 3Cl : 3Na, 3O, 3H, 1Fe, 1Cl)
This is the final balanced equation. Note that it is usually easiest to manipulate oxygen and hydrogen last, since they are often involved in multiple molecules.
To balance an equation, we need to make sure there is the same amount of elements to the left of the arrow as there is to the right. We also need all the charges to balance out. We notice right away that there are three chlorine atoms on the left, but only one on the right.

We can solve this by multiplying NaCl by three.

This causes us to have an imbalance of sodium, which we can correct by manipulating NaOH.

This is the final balanced equation. Note that it is usually easiest to manipulate oxygen and hydrogen last, since they are often involved in multiple molecules.
← Didn't Know|Knew It →
Calcium hydroxide is treated with hydrochloric acid to produce water and calcium chloride. Write a balanced chemical reaction that describes this process.
Calcium hydroxide is treated with hydrochloric acid to produce water and calcium chloride. Write a balanced chemical reaction that describes this process.
Tap to reveal answer
Calcium is in the second group of the periodic table, and is therefore going to have a  oxidation number. Hydroxide ions have a
oxidation number. Hydroxide ions have a  charge. Calcium hydroxide will have the formula
charge. Calcium hydroxide will have the formula  .
.
Chloride ions have a  charge and hydrogen ions have a
charge and hydrogen ions have a  charge. The formula for hydrochloric acid is
charge. The formula for hydrochloric acid is  .
.
On the products side, water has the formula  and calcium chloride has the formula
and calcium chloride has the formula  .
.
Now that we know all of the formulas, we can write our reaction:

In order to balance the chloride atoms, we need to add coefficients.

Calcium is in the second group of the periodic table, and is therefore going to have a 


Chloride ions have a 


On the products side, water has the formula 

Now that we know all of the formulas, we can write our reaction:
In order to balance the chloride atoms, we need to add coefficients.
← Didn't Know|Knew It →

In the balanced version of the preceding equation, what is the coefficient of nitrogen dioxide?
In the balanced version of the preceding equation, what is the coefficient of nitrogen dioxide?
Tap to reveal answer
In the balanced version of the equation:

Nitrogen dioxide's coefficient is 6 in order to balance the 6 moles of nitrogen provided by the 6 moles of nitric acid on the equation's left side.
In the balanced version of the equation:
Nitrogen dioxide's coefficient is 6 in order to balance the 6 moles of nitrogen provided by the 6 moles of nitric acid on the equation's left side.
← Didn't Know|Knew It →
Transesterification is an industrially important process for the production of biodiesel fuel (that most diesel engines can run cleanly on) and glycerin from triglycerides (usually people use vegetable oil as their feedstock).
One easy to do and high yielding method involves the reaction of ethanol or methanol with sodium hydroxide to produce the super bases sodium ethoxide or sodium methoxide respectively. The super base is then used in the transesterification reaction with triglycerides to produce glycerin and ethyl or methyl esters (biodiesel). The products can easily be separated by differences in density. Note: For this reaction to occur the reactants must be free of water as when water is present saponification occurs (how soap is made) which will compete with the transesterification reaction. Note: just because the reaction described above is easy doesn't mean that it is anywhere near safe; please do thoroughly research any reaction you plan to undertake and take all possible safety precautions. That being said, this process is becoming much more popular lately and with the recent focus on alternative energy may well soon account for a decent portion of fuel production.
Catalyst formation equation:

Unbalanced transesterification equation:
![RCO_2CH_2CH(O_2CR)CH_2CO_2R +CH_3OH \xrightarrow[]{Catalyst} C_3H_8O_3+CH_3CO_2R](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/522045/gif.latex)
Balance the following transesterification reaction assuming that all R-groups are identical:
![RCO_2CH_2CH(O_2CR)CH_2CO_2R +CH_3OH \xrightarrow[]{Catalyst} C_3H_8O_3+CH_3CO_2R](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/522044/gif.latex)
Transesterification is an industrially important process for the production of biodiesel fuel (that most diesel engines can run cleanly on) and glycerin from triglycerides (usually people use vegetable oil as their feedstock).
One easy to do and high yielding method involves the reaction of ethanol or methanol with sodium hydroxide to produce the super bases sodium ethoxide or sodium methoxide respectively. The super base is then used in the transesterification reaction with triglycerides to produce glycerin and ethyl or methyl esters (biodiesel). The products can easily be separated by differences in density. Note: For this reaction to occur the reactants must be free of water as when water is present saponification occurs (how soap is made) which will compete with the transesterification reaction. Note: just because the reaction described above is easy doesn't mean that it is anywhere near safe; please do thoroughly research any reaction you plan to undertake and take all possible safety precautions. That being said, this process is becoming much more popular lately and with the recent focus on alternative energy may well soon account for a decent portion of fuel production.
Catalyst formation equation:
Unbalanced transesterification equation:
Balance the following transesterification reaction assuming that all R-groups are identical:
Tap to reveal answer
The steps for balancing equations are as follows and should be done in order:
1.) Check for diatomic molecules: In this case there are none so move on to step 2
2.) Balance the metals (hydrogen is not considered a metal in this application): We have no metals in our equation, so move on to step 3 (the sodium is part of the catalyst so we don't consider it part of the balancing).
3.) Balance the nonmetals (not including oxygen). If you notice the left hand side of the equation has 3R groups and the right hand side only has one R-group. To fix this we can put a coefficient of 3 in front of  on the right hand side giving us the following:
on the right hand side giving us the following:
![RCO_2CH_2CH(O_2CR)CH_2CO_2R +CH_3OH \xrightarrow[]{Catalyst} C_3H_8O_3+3CH_3CO_2R](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/522046/gif.latex)
On the left hand side of the equation we have 7 carbon atoms but on the right hand side we have 9 carbon atoms. This can be rectified by putting a coefficient of 3 in front of  on the left hand side of the equation giving us the following:
on the left hand side of the equation giving us the following:
![RCO_2CH_2CH(O_2CR)CH_2CO_2R +3CH_3OH \xrightarrow[]{Catalyst} C_3H_8O_3+3CH_3CO_2R](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/522047/gif.latex)
Now all the nonmetals (excluding oxygen) have been balanced and we can move on to step 4
4.) Balance oxygen. There are 9 oxygen atoms on both sides of the equation. Move on to step 5
5.) Balance Hydrogen. There are 17 hydrogens on both sides of the equation.
6.) Recount all atoms to make sure you have balanced correctly. 9 carbons on both sides, 3 R-groups on both sides, 9 oxygen on both sides, and 17 hydrogens on both sides.
If this problem had involved ionic species you would also want to make certain that the charges are balanced on both sides of the equation.
The charges on both sides add up to zero so your equation is balanced:
![RCO_2CH_2CH(O_2CR)CH_2CO_2R +3CH_3OH \xrightarrow[]{Catalyst} C_3H_8O_3+3CH_3CO_2R](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/522048/gif.latex)
The steps for balancing equations are as follows and should be done in order:
1.) Check for diatomic molecules: In this case there are none so move on to step 2
2.) Balance the metals (hydrogen is not considered a metal in this application): We have no metals in our equation, so move on to step 3 (the sodium is part of the catalyst so we don't consider it part of the balancing).
3.) Balance the nonmetals (not including oxygen). If you notice the left hand side of the equation has 3R groups and the right hand side only has one R-group. To fix this we can put a coefficient of 3 in front of 
On the left hand side of the equation we have 7 carbon atoms but on the right hand side we have 9 carbon atoms. This can be rectified by putting a coefficient of 3 in front of 
Now all the nonmetals (excluding oxygen) have been balanced and we can move on to step 4
4.) Balance oxygen. There are 9 oxygen atoms on both sides of the equation. Move on to step 5
5.) Balance Hydrogen. There are 17 hydrogens on both sides of the equation.
6.) Recount all atoms to make sure you have balanced correctly. 9 carbons on both sides, 3 R-groups on both sides, 9 oxygen on both sides, and 17 hydrogens on both sides.
If this problem had involved ionic species you would also want to make certain that the charges are balanced on both sides of the equation.
The charges on both sides add up to zero so your equation is balanced:
← Didn't Know|Knew It →
Consider the following unbalanced reaction:

Once this equation has been balanced, what are the the respective stoichiometric coefficients (listed in the order in which the above compounds appear in the reaction)?
Consider the following unbalanced reaction:
Once this equation has been balanced, what are the the respective stoichiometric coefficients (listed in the order in which the above compounds appear in the reaction)?
Tap to reveal answer
To balance chemical reactions, it's usually more effective to save the more common elements, such as oxygen, for last. For this reaction, let's go ahead and start by balancing the element  on both sides.
on both sides.

Because there are  moles of
moles of  on the right side, we'll need to have
on the right side, we'll need to have  moles of
moles of  . Next, let's go ahead and balance nitrogen on both sides.
. Next, let's go ahead and balance nitrogen on both sides.

Since we added a coefficient of  to the
to the  in the first step, there are now
in the first step, there are now  moles of
moles of  on the left. Thus, we'll need to add a coefficient of
on the left. Thus, we'll need to add a coefficient of  to the
to the  . Next, if we check the amount of
. Next, if we check the amount of  ,
,  , and
, and  on both sides, we see that they are equal. Thus, we can recognize that
on both sides, we see that they are equal. Thus, we can recognize that  and
and  have coefficients of
have coefficients of  , and do not need to be altered.
, and do not need to be altered.
Our final balanced equation looks like this.

To balance chemical reactions, it's usually more effective to save the more common elements, such as oxygen, for last. For this reaction, let's go ahead and start by balancing the element 
Because there are 



Since we added a coefficient of 











Our final balanced equation looks like this.
← Didn't Know|Knew It →
Balance the following equation:
FeCl3 + NOH5 → Fe(OH)3 + NH4Cl
Balance the following equation:
FeCl3 + NOH5 → Fe(OH)3 + NH4Cl
Tap to reveal answer
The first thing to do is to balance the Cl (3 on the left; one on the right) ← add 3 for NH4Cl
Now, there are 3 N on the right and only one on the left; add a 3 to the NOH5
Check to see that the Fe, H, and O balance, which they do
The first thing to do is to balance the Cl (3 on the left; one on the right) ← add 3 for NH4Cl
Now, there are 3 N on the right and only one on the left; add a 3 to the NOH5
Check to see that the Fe, H, and O balance, which they do
← Didn't Know|Knew It →
Balance the following equation:
NaOH + H2SO4 → H2O + Na2SO4
Balance the following equation:
NaOH + H2SO4 → H2O + Na2SO4
Tap to reveal answer
Balancing equations:
there is 1 NaOH and 1 H2SO4 in the reactants; and 1 H2O and 1 Na2SO4 in the products
steps:
-
balance the Na first; get 2 NaOH
-
balance H next; get 1 H2SO4, 2 H2O
-
check to make sure that O and S balance
Balancing equations:
there is 1 NaOH and 1 H2SO4 in the reactants; and 1 H2O and 1 Na2SO4 in the products
steps:
-
balance the Na first; get 2 NaOH
-
balance H next; get 1 H2SO4, 2 H2O
-
check to make sure that O and S balance
← Didn't Know|Knew It →
_Fe2O3 + _HCl ⇌ _FeCl3 + _H2O
The Following question will be based on the unbalanced reaction above
For the reaction above to be balanced what coefficient should be in front of the compound HCl?
_Fe2O3 + _HCl ⇌ _FeCl3 + _H2O
The Following question will be based on the unbalanced reaction above
For the reaction above to be balanced what coefficient should be in front of the compound HCl?
Tap to reveal answer
Balancing reactions is best acheived by using a stepwise approach. It is useful to work through each atom making sure it is present in a balanced fashion on both sides of the equation. Starting with Fe, Fe2O3 is the only molecule with Fe present on the left side of the equation and FeCl3 is the only molecule with Fe present on the right side of the equation. Thus the molar ratio of Fe2O3 to FeCl3 must be 1:2. Moving on to O, the O on the left side of the equation exists as Fe2O3 and H2O on the right. The molar ratio of Fe2O3 to H2O is therefore 1:3. Cl exists in HCl on the left and FeCl3 on the right. Thus the molar ratio of HCl to FeCl3 must be 3:1. To ensure that these molar ratios are maintaned the balanced formula is then determined to be Fe2O3 + 6HCl -> 2FeCl3 + 3H2O
Balancing reactions is best acheived by using a stepwise approach. It is useful to work through each atom making sure it is present in a balanced fashion on both sides of the equation. Starting with Fe, Fe2O3 is the only molecule with Fe present on the left side of the equation and FeCl3 is the only molecule with Fe present on the right side of the equation. Thus the molar ratio of Fe2O3 to FeCl3 must be 1:2. Moving on to O, the O on the left side of the equation exists as Fe2O3 and H2O on the right. The molar ratio of Fe2O3 to H2O is therefore 1:3. Cl exists in HCl on the left and FeCl3 on the right. Thus the molar ratio of HCl to FeCl3 must be 3:1. To ensure that these molar ratios are maintaned the balanced formula is then determined to be Fe2O3 + 6HCl -> 2FeCl3 + 3H2O
← Didn't Know|Knew It →
After the following reaction is balanced, how many moles of H2O can 4 moles of C2H6 produce?
__C2H6(s) + O2(g) → H2O(l) + CO2(g)
After the following reaction is balanced, how many moles of H2O can 4 moles of C2H6 produce?
__C2H6(s) + O2(g) → H2O(l) + CO2(g)
Tap to reveal answer
The reaction balances out to 2C2H6(s) + 7O2(g) → 6H2O(l) + 4CO2(g). For every 2 moles of C2H6 you can produce 6 moles of H2O. Giving you a total production of 12 moles when you have 4 moles of C2H6.
The reaction balances out to 2C2H6(s) + 7O2(g) → 6H2O(l) + 4CO2(g). For every 2 moles of C2H6 you can produce 6 moles of H2O. Giving you a total production of 12 moles when you have 4 moles of C2H6.
← Didn't Know|Knew It →
Consider the following unbalanced equation for the combustion of propane,  :
:

If you were to combust one mole of propane, how many moles of water would you produce?
Consider the following unbalanced equation for the combustion of propane, 
If you were to combust one mole of propane, how many moles of water would you produce?
Tap to reveal answer
Begin by balancing the equation. There are many ways to do this, but one method that is particularly useful is to assume that you have 1 mole of your hydrocarbon (propane), and balance the equation from there. It may be necessary to manipulate an equation further if you end up with fractions, but all you will need to do is multiply by an integer if that is the case.
First, we will balance the carbons. There are three carbons in propane, so we will make sure there are three carbons on the right side of the arrow as well:

This is not complete. We will next balance the hydrogens. There are eight hydrogens on the left side of the equation, so:

The last step is to balance the oxygens on the left and right side of the equation

Our equation is balanced and all coefficients are integers. If we begin with one mole of propane, we will produce four moles of water.
Begin by balancing the equation. There are many ways to do this, but one method that is particularly useful is to assume that you have 1 mole of your hydrocarbon (propane), and balance the equation from there. It may be necessary to manipulate an equation further if you end up with fractions, but all you will need to do is multiply by an integer if that is the case.
First, we will balance the carbons. There are three carbons in propane, so we will make sure there are three carbons on the right side of the arrow as well:
This is not complete. We will next balance the hydrogens. There are eight hydrogens on the left side of the equation, so:
The last step is to balance the oxygens on the left and right side of the equation
Our equation is balanced and all coefficients are integers. If we begin with one mole of propane, we will produce four moles of water.
← Didn't Know|Knew It →
Consider the following unbalanced equation:

How many grams of solid iron are needed to make 36.0g of  ? Assume that chlorine is in excess.
? Assume that chlorine is in excess.
Consider the following unbalanced equation:
How many grams of solid iron are needed to make 36.0g of 
Tap to reveal answer

First, we will balance the equation:

Since chlorine is in excess, we know that the limiting reagent is iron.

First, we will balance the equation:
Since chlorine is in excess, we know that the limiting reagent is iron.
← Didn't Know|Knew It →
Consider the following reaction:

When the equation is balanced, what will be the coefficient in front of HCl?
Consider the following reaction:
When the equation is balanced, what will be the coefficient in front of HCl?
Tap to reveal answer
When balancing equations, the goal is to make sure that the same atoms, in both type and amount, are on both the reactant and product side of the equation. A helpful approach is to write down the number of atoms already on both sides of the unbalanced equation. This way, you can predict which compounds need to be increased on which side in order to balance the equation. It also helps to balance oxygen and hydrogen last in the equation.
In this reaction, we can balance as follows.
Reactants: 1K, 1Mn, 1Cl, 4O, 1H
Products: 1K, 1Mn, 5Cl, 1O, 2H
So, we will need to increase H2O and HCl. The final balanced equation is written below.

When balancing equations, the goal is to make sure that the same atoms, in both type and amount, are on both the reactant and product side of the equation. A helpful approach is to write down the number of atoms already on both sides of the unbalanced equation. This way, you can predict which compounds need to be increased on which side in order to balance the equation. It also helps to balance oxygen and hydrogen last in the equation.
In this reaction, we can balance as follows.
Reactants: 1K, 1Mn, 1Cl, 4O, 1H
Products: 1K, 1Mn, 5Cl, 1O, 2H
So, we will need to increase H2O and HCl. The final balanced equation is written below.
← Didn't Know|Knew It →